In the realm of organic chemistry, a systematic and unambiguous naming system is crucial. This is where the IUPAC (International Union of Pure and Applied Chemistry) nomenclature comes into play. It provides a standardized set of rules for naming organic compounds, ensuring clarity and consistency in scientific communication across the globe. For alkanes, the simplest type of hydrocarbons, the IUPAC system offers a straightforward approach to assigning unique and informative names. Understanding these rules is fundamental for any student delving into organic chemistry.
Understanding the Basics: Parent Chains and Alkyl Groups
The foundation of IUPAC nomenclature lies in identifying the longest continuous chain (LCC) of carbon atoms within a molecule. This LCC serves as the parent chain, and its length dictates the base name of the alkane. The number of carbon atoms in this chain is indicated by specific stems, as outlined in Table 1.3.
Table 1.3: Stems for Carbon Chains in IUPAC Nomenclature
| Stem | Number of Carbon Atoms |
|---|---|
| meth- | 1 |
| eth- | 2 |
| prop- | 3 |
| but- | 4 |
| pent- | 5 |
| hex- | 6 |
| hept- | 7 |
| oct- | 8 |
| non- | 9 |
| dec- | 10 |
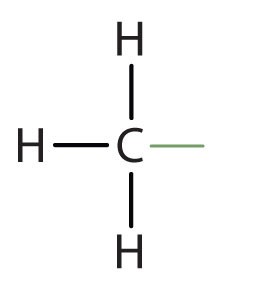
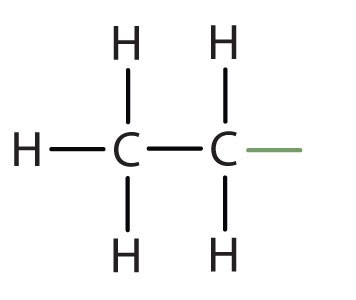
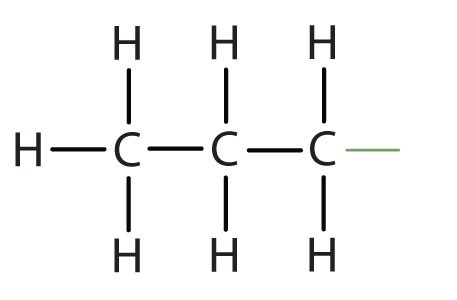
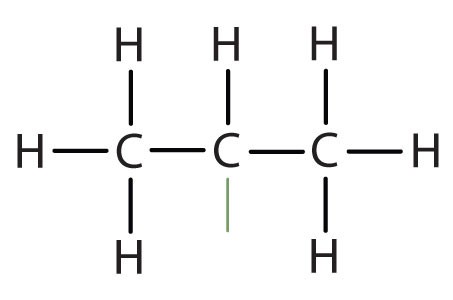
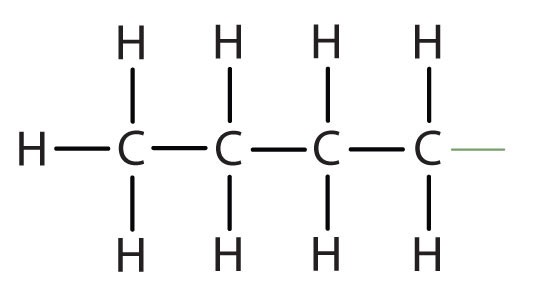
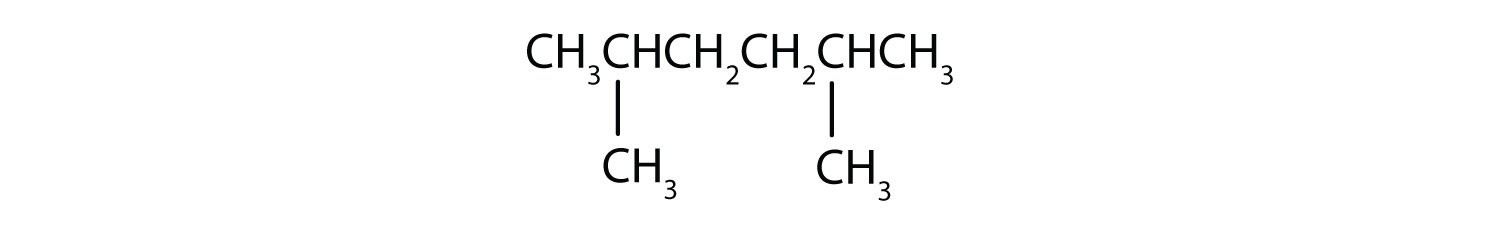
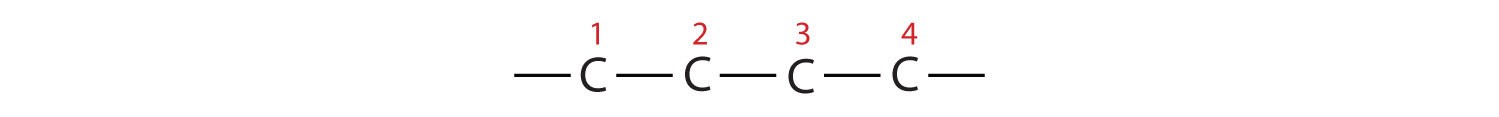
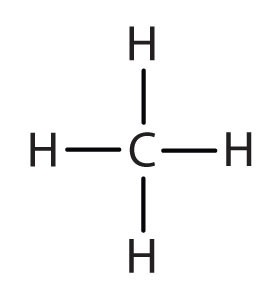
When hydrogen atoms are removed from an alkane, we get alkyl groups. These groups act as substituents, branching off from the parent chain. To name an alkyl group, we replace the -ane suffix of the parent alkane with -yl. For instance, methane (CH4) becomes a methyl group (CH3–). Common alkyl groups are listed in Table 1.4.
Table 1.4: Common Alkyl Groups
| Parent Alkane | Image | Alkyl Group | Image | Condensed Formula |
|---|---|---|---|---|
| methane | CH3– | |||
| ethane | CH3CH2– | |||
| propane | CH3CH2CH2– | |||
| isopropyl | CH3– | |||
| butane | CH3CH2CH2CH2– |
*Note: There are multiple butyl groups, but we will focus on the most common one here.
Step-by-Step Guide to IUPAC Nomenclature for Alkanes
Naming alkanes using the IUPAC system involves a series of logical steps. Let’s break down the process:
Step 1: Identify the Longest Continuous Chain (LCC)
Find the longest continuous chain of carbon atoms in the molecule. This chain is not always straight and might bend. The number of carbons in this LCC determines the parent alkane name using the stems from Table 1.3 and adding the suffix -ane.
Step 2: Number the Carbon Atoms
Number the carbon atoms in the LCC starting from the end that gives the substituents the lowest possible numbers. This ensures that the positions of substituents are indicated by the smallest numbers.
Step 3: Identify and Name Substituent Groups
Identify any alkyl groups attached to the parent chain. Name these substituents using the -yl suffix as discussed earlier (e.g., methyl, ethyl, propyl).
Step 4: Arrange Substituents Alphabetically
List the names of the substituent groups in alphabetical order before the parent alkane name. Prefixes like di- , tri- , tetra- (used for multiple identical substituents) are not considered when alphabetizing. For example, “ethyl” comes before “dimethyl”.
Step 5: Construct the Full IUPAC Name
Combine the numbered positions of the substituents, the substituent names (in alphabetical order), and the parent alkane name into a single name. Use hyphens to separate numbers from substituent names and commas to separate numbers from each other. The substituent names and parent alkane name are written as one word.
Examples: Naming Alkanes
Let’s apply these rules to name some alkanes:
Example 1:
- LCC: The longest continuous chain has five carbon atoms, so the parent chain is pentane.
- Numbering: Numbering from left to right gives the methyl group at position 2. Numbering from right to left would give position 4, which is higher. So, we number from left to right.
- Substituent: There is a methyl group (CH3–) attached to the parent chain.
- Alphabetical Order: Only one substituent, so no alphabetization needed.
- IUPAC Name: 2-methylpentane
Example 2:
- LCC: The longest continuous chain has six carbon atoms, making it hexane.
- Numbering: Numbering from either direction yields methyl groups at positions 2 and 5.
- Substituents: Two methyl groups.
- Alphabetical Order: Only methyl groups, no alphabetization needed. Use the prefix “di-” for two.
- IUPAC Name: 2,5-dimethylhexane
Example 3:
- LCC: The longest chain has eight carbon atoms, so the parent chain is octane.
- Numbering: Numbering from the right gives substituents at position 4. Numbering from the left would give position 5. Therefore, number from the right.
- Substituents: An ethyl group (CH3CH2–) and a methyl group (CH3–), both attached to the 4th carbon atom.
- Alphabetical Order: “Ethyl” comes before “methyl”.
- IUPAC Name: 4-ethyl-4-methyloctane
Examples: Drawing Alkanes from IUPAC Names
The IUPAC system also allows us to draw the structure of an alkane from its name. Here’s how:
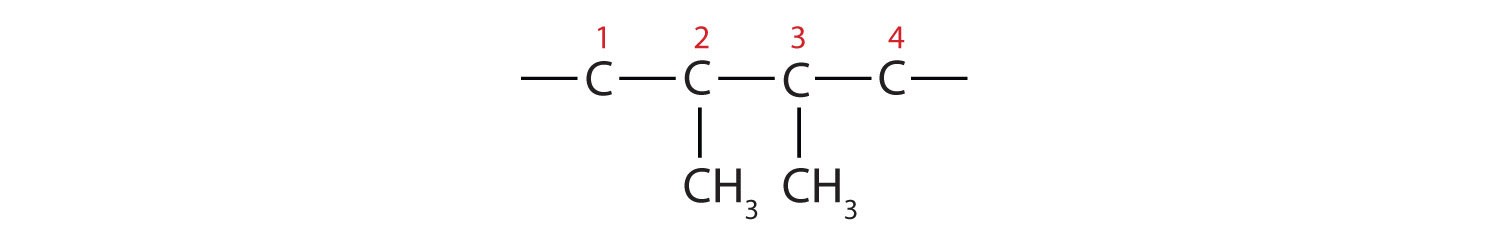
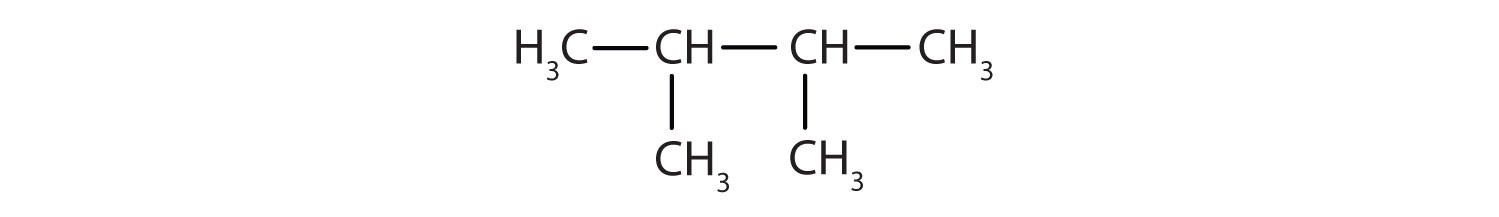
Example 1: 2,3-dimethylbutane
- Parent Chain: “Butane” indicates a four-carbon parent chain. Draw four carbon atoms in a row: –C–C–C–C–
- Substituents: “2,3-dimethyl” indicates two methyl groups (CH3–) attached to carbons 2 and 3 of the butane chain. Add these methyl groups:
- Complete with Hydrogen: Add hydrogen atoms to each carbon to ensure each carbon has four bonds.
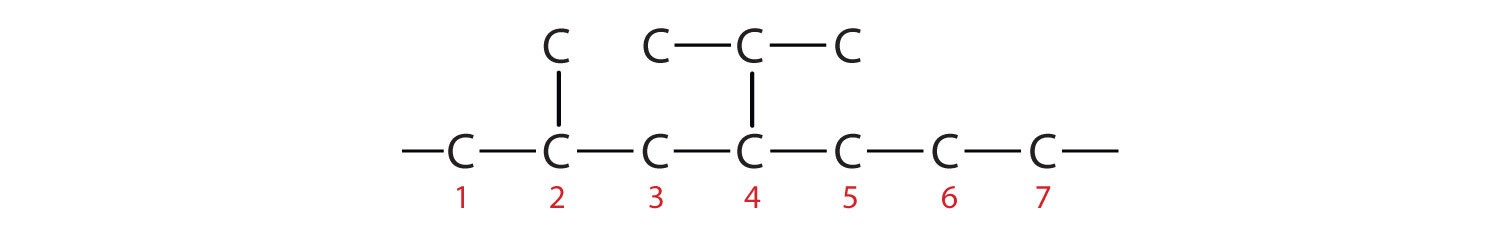
Example 2: 4-ethyl-2-methylheptane
- Parent Chain: “Heptane” means a seven-carbon chain: –C–C–C–C–C–C–C–
- Substituents: “4-ethyl” and “2-methyl” indicate an ethyl group on carbon 4 and a methyl group on carbon 2. Add these groups:
- Complete with Hydrogen: Fill in hydrogens to complete four bonds for each carbon.
Key Takeaways
- IUPAC nomenclature provides a systematic way to name alkanes, ensuring clear and consistent communication in chemistry.
- The key steps involve identifying the longest continuous carbon chain, numbering the chain, naming and alphabetizing substituents, and combining these elements into a single IUPAC name.
- Mastering IUPAC nomenclature is essential for understanding and communicating organic chemistry concepts effectively.