This year’s Nobel Prize for the CRISPR/Cas system highlights a crucial scientific journey that began with the groundbreaking discovery of Guide Rnas (gRNAs) in the 1990s. This foundational work underscores how major scientific breakthroughs are often built upon preceding discoveries.
Subject Categories: S&S: History & Philosophy of Science, RNA Biology
The CRISPR-Cas9 system has become indispensable in molecular biology. In nature, it serves as an adaptive immune system in bacteria, enabling them to remember and defend against past viral infections. However, CRISPR-Cas9 is most celebrated as a remarkably versatile and precise gene-editing tool. This transformative application earned Emmanuelle Charpentier and Jennifer Doudna the Nobel Prize in Chemistry in 2020 (Jinek, Chylinski et al, 2012). At the core of this system is Cas9, a programmable endonuclease that targets specific DNA sequences, creating double-strand breaks that can be harnessed for diverse gene modification techniques. The specificity of Cas9 is dictated by a small RNA molecule known as guide RNA (gRNA). This gRNA base pairs with the target DNA sequence and simultaneously interacts with Cas9 to initiate the site-specific DNA cleavage (Fig. 1). Interestingly, the discovery of the first guide RNAs and the fundamental principle of RNA-directed protein activity at specific nucleic acid sequences predates the CRISPR-Cas9 revolution by over two decades.
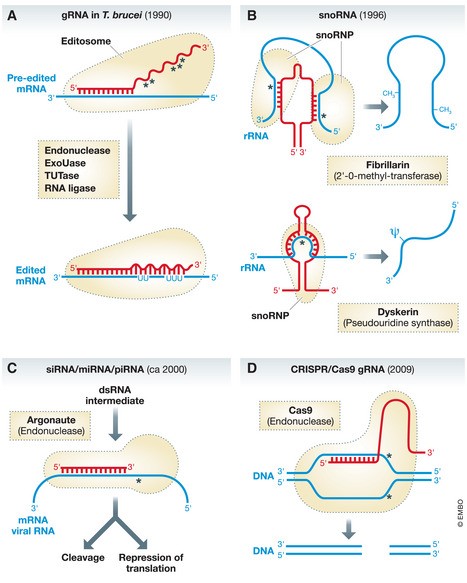
Figure 1. Chronological Overview of “Guide RNAs” and Their Diverse Roles.
(A) Guide RNAs in Trypanosomes and Mitochondrial RNA Editing: These gRNAs direct uridine insertion and deletion in pre-edited RNA within mitochondria. This process involves a cascade of enzymatic activities: endoribonuclease, terminal uridyltransferase (Tutase), U-specific exoribonuclease (ExoUase), and RNA ligase. The wavy red line indicates the gRNA region responsible for guiding RNA editing. (B) SnoRNAs in rRNA Modification: Small nucleolar RNAs (snoRNAs) guide two types of ribosomal RNA (rRNA) modifications: 2’-O-methylation and pseudouridylation. Different sets of snoRNAs are responsible for each modification type. (C) siRNA and miRNA in Gene Regulation: Small interfering RNAs (siRNAs) and microRNAs (miRNAs) are processed through distinct pathways, resulting in double-stranded RNA intermediates. The sense strand of these intermediates is incorporated into protein complexes containing Argonaute family proteins. siRNAs and miRNAs regulate gene expression by inducing target RNA degradation or by modulating translation and transcription. (D) CRISPR/Cas9 System for Targeted DNA Cleavage: The CRISPR/Cas9 system utilizes a guide RNA (gRNA) to direct Cas9 endonuclease to a specific DNA sequence, resulting in a double-strand break. This system has revolutionized genetic engineering across various organisms. The optimized system shown here features a fusion of the gRNA and tracRNA. In the native prokaryotic CRISPR-Cas system, Cas9 associates with individual gRNA and tracRNA molecules to provide adaptive immunity against viral infections. Red regions represent “guide RNA” segments; blue indicates the “gRNA” target sequence; broken black lines depict proteins or protein complexes associated with “gRNA.” Base pairing regions between gRNA and target nucleic acids are highlighted. Asterisks mark the sites of cleavage or modification in the target nucleic acid.
… the discovery of the first gRNAs […] predates the discovery of the CRISPR/Cas9 system by more than two decades.
This article delves into the history of the term “guide RNA,” tracing its origins and the evolution of the concept. While not directly involved in these initial discoveries, the author shares personal insights, particularly regarding Beat Blum, whose seminal 1990 paper first coined the term gRNA (Blum et al, 1990). The discovery of gRNAs by Beat Blum serves as a compelling illustration of the often complex and winding paths that lead to scientific breakthroughs, making his story a central focus here.
Unraveling RNA Editing: The Genesis of Guide RNA
The narrative begins with Rob Benne at the University of Amsterdam, who was investigating the mitochondrial DNA and corresponding mRNAs of Trypanosoma brucei, a parasitic protozoan. Benne’s analysis of cytochrome oxidase subunit 2 (Cox2) revealed a startling discrepancy: the genomic DNA sequence and the mRNA-derived sequence were not identical. He consistently observed a four-uridine insertion in the mRNA that was absent in the gene. This insertion corrected a frameshift predicted in the Cox2 gene. Initially met with disbelief, Benne’s findings, published in 1986, proposed a novel process termed RNA editing. This process precisely inserted four uridines at a specific position in the Cox2 transcript to generate a functional, translatable mRNA (Benne et al, 1986).
Skepticism initially surrounded Benne’s paper, but this gradually dissipated as further examples of RNA editing, even more dramatic ones, were discovered, all within trypanosomatids. These editing events involved multiple uridine insertions and deletions, so extensive in some cases that the mature mRNAs no longer hybridized to their corresponding genes. RNA editing gained traction within the scientific community, prompting numerous reviews discussing its implications. The most puzzling question was the source of the information for these edited sequences. Despite extensive searches, no DNA or RNA template matching the edited sequences could be found in the trypanosome mitochondrial genome. This led some to believe that RNA editing might challenge Francis Crick’s central dogma of molecular biology, sparking unconventional theories about the underlying mechanisms.
… some people thought that RNA editing would challenge the central dogma of Francis Crick, which led to some quite far-fetched ideas of how the process may work.
The Emergence of the First Guide RNAs
During this period, Beat Blum and the author were PhD students in Thomas Seebeck’s lab at the University of Bern, both working with T. brucei. Beat was captivated by the newly discovered RNA editing phenomenon. After completing his PhD, he pursued a postdoctoral position with Larry Simpson at UCLA, a leading expert in the field. Beat secured a postdoctoral fellowship from the Swiss National Science Foundation (SNF) with an ambitious proposal: to uncover the source of information guiding RNA editing. Unlike others, Beat remained committed to the central dogma and hypothesized that the solution lay in specific base pairing with nucleic acids complementary to the edited sequences. His SNF proposal detailed various strategies to identify these hypothetical nucleic acids. Finding this missing piece of the RNA editing puzzle became Beat’s unwavering mission even before he joined Simpson’s lab. He confidently declared to anyone who would listen – and many who wouldn’t – that he would go to Larry Simpson’s lab and solve the RNA editing mystery once and for all. At the time, many, including the author, were skeptical.
… RNA editing became Beat’s mission and he told everybody who wanted to hear it – and many who did not – that he will [..] solve the problem of RNA editing once and for all.
However, shortly after arriving at Larry Simpson’s lab, Beat made a significant breakthrough. He performed a computational analysis and identified short sequences in the mitochondrial genome of Leishmania tarentolae, another trypanosomatid, that exhibited striking similarity to segments of the edited mRNAs. These sequences were also flanked on their 3′ side by short stretches closely matching the unedited regions of the same mRNA. Although others had attempted similar approaches using available sequence data, they had been unsuccessful. Beat’s key innovation was allowing for guanine-uracil (G-U) base pairs in his analysis. G-U base pairing was a known phenomenon, and its critical role in RNA function was highlighted in a review published in the inaugural issue of EMBO Reports (Varani & McClain, 2000).
Beat subsequently demonstrated that these sequences matching the edited regions were indeed transcribed into short RNAs. In January 1990, he, Larry Simpson, and Norbert Bakalara published these findings in Cell (Blum et al, 1990), introducing the term “guide RNAs” – the first documented use of this term in scientific literature. They proposed a model where the 5′ portions of gRNAs hybridize with the regions flanking the pre-edited mRNA, thus guiding the RNA editing machinery to the precise editing sites. This concept, RNA-guided targeting of enzymatic activity, is the essence of what is now broadly recognized as the gRNA concept.
It is important to note that trypanosomal gRNAs are not only the first discovered but also the most complex known to date. They orchestrate the RNA editing machinery – encompassing endonuclease, terminal uridyltransferase, U-specific exoribonuclease, and RNA ligase activities – to the correct location on the pre-edited mRNA. These gRNAs then instruct these enzymes to sequentially insert or delete the precise number of uridines (Fig. 1). Today, we understand that RNA editing, the post-transcriptional modification of mRNA coding sequences, is widespread and diverse. However, remarkably, RNA editing in trypanosomatid mitochondria remains the only known instance that relies on guide RNAs.
SnoRNAs: Another Class of Guide RNAs
Six years after the discovery of trypanosomal gRNAs, another class of small RNAs, small nucleolar RNAs (snoRNAs), were also found to function as guide RNAs. SnoRNAs, known for many years to reside in the nucleolus, were shown by Zsuzsanna Kiss-Laszlo to guide the 2′-O-methylation of specific nucleotides in ribosomal RNAs (rRNAs) (Kiss-Laszlo, Henry et al, 1996). A year later, the same group demonstrated that a different set of snoRNAs directed the site-specific conversion of uridines to pseudouridines in rRNA. Eukaryotic cells contain hundreds of distinct snoRNAs, many encoded within introns. These snoRNAs, along with associated proteins including modification enzymes, form ribonucleoprotein particles (RNPs) called snoRNPs. Each snoRNA contains a nucleotide sequence complementary to the region surrounding the nucleotide to be modified in the rRNA target (Fig 1). This complementarity enables snoRNAs to recognize their target RNAs and recruit modification enzymes to the correct location. The discovery that snoRNAs guide nucleotide modifications was surprising, as bacterial rRNA modifications, while common, do not require guide RNAs.
Over subsequent decades, the functions of numerous snoRNA-like guide RNAs have been elucidated. They are now known to guide site-specific modifications in small nuclear RNAs (snRNAs) involved in splicing and, in some cases, transfer RNAs (tRNAs). A subgroup of snoRNAs also plays a role in the structural reorganization of rRNA before processing, indicating functions beyond nucleotide modification. Furthermore, vertebrate telomerase contains a snoRNA-type RNA subunit, TERC, which serves as a template for telomere elongation. Given the vast number of snoRNA-like RNAs with yet-undetermined functions in eukaryotes, it is highly probable that further novel roles for these molecules will be discovered in the future.
Considering the great number of snoRNA-like RNAs, with as yet unassigned functions found in eukaryotes, it is likely that even more novel roles for these molecules will be discovered in future.
The Expanding Universe of Guide RNAs
Following the groundbreaking discoveries of the 1990s, the early 2000s ushered in the discovery of a vast universe of novel small RNAs. These RNAs, including small interfering RNAs (siRNAs), microRNAs (miRNAs), and PIWI-interacting RNAs (piRNAs), function as guide RNAs, directing protein complexes to specific RNA targets (reviewed in Bartel, 2004; Czech, et al, 2018; Fig 1). siRNAs originate from long double-stranded RNA (dsRNA), which can be formed from endogenous sense and antisense transcripts or introduced exogenously from viruses or synthetic sources. miRNAs and piRNAs are processed from larger precursor RNAs through distinct and complex maturation pathways. The processing of siRNAs and miRNAs generates short, double-stranded RNA (dsRNA) intermediates. Subsequently, the guide strand of the dsRNA, which is complementary to the target RNA, is incorporated into a protein complex containing an Argonaute family protein member. Many Argonaute proteins possess endonuclease activity. In the case of siRNAs with perfect complementarity to their targets, Argonaute proteins cleave the target RNAs at the site dictated by the siRNA. When complementarity is imperfect, as often seen with miRNAs targeting the 3′ untranslated region (3’UTR) of mRNAs, the outcome is translational repression or, in some instances, translational activation. Although piRNAs do not form double-stranded intermediates, they associate with a specific subfamily of Argonaute proteins. Their primary function is to silence transposon activity in germline cells through both transcriptional and post-transcriptional mechanisms.
In essence, these small guide RNA-like molecules play diverse roles in gene regulatory processes. Their specificity is ensured by base pairing with their target RNAs. They can guide target RNA degradation, modulate transcription or translation, or influence epigenetic states through various mechanisms.
Standing on the Shoulders of Giants: RNA Splicing and the gRNA Concept
Was Beat Blum, the discoverer of trypanosomal gRNAs, also the originator of the broader gRNA concept? As is often the case in science, fundamental discoveries are built upon incremental steps and rarely represent the revolutionary leaps they might initially appear to be. This holds true for the gRNA concept as well. The importance of RNA-RNA interactions in positioning enzymatic activities to specific sites on target RNAs was already evident in mRNA splicing. mRNA splicing is a complex and dynamic process involving numerous rearrangements of small nuclear RNAs (snRNAs) and their associated proteins. It proceeds through two transesterification steps. Step 1 results in a free 5′ exon and an intron lariat – 3′ exon intermediate linked to the branch point, a conserved sequence within the intron. Step 2 subsequently joins the 5′ exon with the 3′ exon. Both steps are ultimately catalyzed by the U6 snRNA. As early as the 1980s, it was recognized that U1 snRNA, which forms the U1 snRNP complex, exhibits complementarity to the 5′ splice site of mRNA precursors (Fig 2; Lerner, Boyle et al, 1980; Rogers & Wall, 1980). In 1986, it was shown that this interaction initiates the splicing cycle (Zhuang & Weiner, 1986). Later in splicing, the branch point sequence is recognized through limited base pairing with U2 snRNA, and the 5′ and 3′ splice sites are again recognized by U5 snRNA. Therefore, recognition of intron boundaries is largely mediated by specific base pairing with various snRNAs, although proteins also contribute significantly. Thus, it is reasonable to argue that early studies on U1 snRNA (Lerner et al, 1980; Rogers & Wall, 1980; Zhuang & Weiner, 1986) represent the conceptual origin of the gRNA concept, even though the extent of complementary base pairing in splicing is generally less extensive than in the smaller guide RNA-like molecules. Beat Blum and his co-authors cited publications from the splicing field in their seminal gRNA paper, suggesting they were likely influenced by these earlier studies.
Figure 2. mRNA Splicing: An Early Example of RNA-Guided Targeting.
U1 snRNA initiates the splicing cycle by base-pairing with the 5′ intron-exon junction of the pre-mRNA. The base-pairing region between U1 snRNA and pre-mRNA is indicated. Subsequently, U2 snRNA recognizes the branchpoint sequence (not shown). More snRNAs and their associated proteins, including U6 snRNA which ultimately catalyzes the two transesterification steps of splicing, assemble around the 5′ and 3′ splice sites, while other snRNAs dissociate from the pre-mRNA. This results in a complex termed the tri-snRNP, containing U2, U5, and U6 snRNAs. This complex catalyzes the first and second splicing steps, leading to the formation of a 5′ exon and an intron lariat – 3′ exon intermediate before the 5′ and 3′ exons are joined. Numerous other dynamic RNA-RNA interactions occur during the splicing cycle that are not depicted here. Red represents U1 snRNA; blue represents (pre-)mRNA; broken black lines represent snRNP complexes.
As it is mostly the case, fundamental discoveries can be traced back to multiple, incremental steps and are only rarely the revolutionary change they appear to be when first published.
Concluding Remarks: Serendipity and the Conservative Approach
Major scientific breakthroughs are often achieved by younger scientists, who may be less constrained by established paradigms and approach problems with fresh perspectives. Beat Blum was 36 when he discovered gRNAs. However, ironically, his breakthrough was not driven by radical, imaginative thinking typically associated with youth. Instead, it was Beat’s unwavering and, in some ways, conservative approach. He stubbornly maintained, against prevailing skepticism, that the mystery of RNA editing information could be resolved through base pairing.
… in my opinion and quite ironically, it was not highly imaginative and innovative thinking that we associate with the young, which led to his breakthrough.
While unconventional thinking might not have been the key, Beat’s deep understanding of the field, including the knowledge that RNA can form G-U base pairs, was crucial. This insight allowed him to overcome the mismatches that had hindered precise alignment of gRNAs and edited mRNA sequences, prompting others to abandon the search for RNA templates in RNA editing.
We now know that RNA editing is a widespread phenomenon, occurring in plant mitochondria and chloroplasts as well, where it involves site-specific C-to-U conversions in numerous primary transcripts and can be extensive. Had RNA editing been first discovered in plants rather than trypanosomes, Beat Blum’s approach might have failed, as RNA editing in plants is not guided by RNAs and base pairing. Instead, site-specific C-to-U conversions are directed by pentatricopeptide repeat proteins that bind RNA in a sequence-specific manner. Thus, as with many scientific discoveries, luck and being in the right place at the right time can play a significant role.
Acknowledgements
The author thanks Sebastian Leidel and Oliver Mühlemann for their critical review of the manuscript. Research in the author’s laboratory is supported by the NCCR “RNA & Disease” and grant 175563, both funded by the Swiss National Science Foundation.
EMBO Reports (2020) 21: e51918.
References
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297
http://www.ncbi.nlm.nih.gov/pubmed/14749165 - Benne R, Van den Burg J, Brakenhoff JP, Tuynman TF, Van Boom JH, & Tromp MC (1986) Major structural differences between mitochondrial mRNAs and kinetoplast DNA from Trypanosoma brucei. Cell 46: 819–826
http://www.ncbi.nlm.nih.gov/pubmed/3736636 - Blum B, Bakalara N, & Simpson L (1990) A model for RNA editing in kinetoplastid mitochondria: “guide” RNA molecules transcribed from maxicircle DNA provide the edited sequence information. Cell 60: 189–198
http://www.ncbi.nlm.nih.gov/pubmed/2296994 - Czech B, Malone CD, Zhou R, & Hannon GJ (2018) piRNA-guided genome defense. Annu Rev Biochem 87: 197–227
http://www.ncbi.nlm.nih.gov/pubmed/29420817 - Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, & Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821
http://www.ncbi.nlm.nih.gov/pubmed/22745249 - Kiss-Laszlo Z, Henry Y, Bachellerie JP, Caizergues-Ferrer M, Kiss T (1996) Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell 85: 1077–1088
http://www.ncbi.nlm.nih.gov/pubmed/8681378 - Lerner MR, Boyle JA, Mount SM, Seidman JG, & Steitz JA (1980) Are snRNPs involved in splicing? Nature 283: 220–224
http://www.ncbi.nlm.nih.gov/pubmed/7354965 - Rogers J, & Wall R (1980) A mechanism for RNA splicing. Proc Natl Acad Sci U S A 77: 1877–1879
http://www.ncbi.nlm.nih.gov/pubmed/6929544 - Varani G, & McClain WH (2000) The G x U wobble base pair. A fundamental building block of RNA structure crucial to RNA function at or near the catalytic center. EMBO Rep 1: 18–23
http://www.ncbi.nlm.nih.gov/pubmed/11256628 - Zhuang Y, & Weiner AM (1986) An ordered two-step mechanism for RNA splicing. Cell 46: 827–835
http://www.ncbi.nlm.nih.gov/pubmed/3736637