1. Introduction
Annually, approximately 47,800 individuals in the United States are diagnosed with HIV, with sexual transmission accounting for about 87% of these cases [1]. Research has consistently shown a strong link between crack/cocaine use and increased sexual risk behavior (SRB) [2–4]. While substance abuse treatment itself contributes to HIV prevention [5], a comprehensive strategy includes HIV education and counseling to empower patients to adopt safer behaviors [6]. However, a 2011 survey revealed that a significant 43% of substance abuse treatment programs in the U.S. do not offer HIV education or counseling [7]. Studies investigating the effectiveness of HIV education in reducing SRB among crack/cocaine users have largely focused on individuals not in treatment [8–10]. Notably, only a couple of studies have assessed HIV education efficacy for cocaine-dependent individuals within treatment settings, and these primarily evaluated knowledge gain rather than SRB reduction [11, 12]. Given the resource constraints in substance treatment programs, HIV education interventions need to be straightforward and require minimal supervision. The key question is whether such simplified HIV education can effectively reduce HIV risk behaviors in a clinically meaningful way. This paper offers preliminary data to address this critical question. Specifically, we analyze data from two clinical trials for cocaine dependence that integrated HIV education alongside cognitive behavioral therapy (CBT). In these pre- and post-intervention evaluations of HIV education and substance abuse treatment on promoting safe sex, we prioritized measures with significant public health implications: complete abstinence or consistent condom use [13]. For comparative context, we also present a combined analysis of two earlier cocaine dependence trials that provided CBT alone, without HIV education.
2. Method
2.1. Participants and Procedures
This study analyzes data from two randomized, placebo-controlled, multisite clinical trials for cocaine dependence to examine changes in SRB before and after interventions. One trial assessed reserpine [14], and the other evaluated tiagabine [15]. Detailed descriptions of the participant demographics and study procedures are available in previous publications [14, 15].
2.2. Study Treatments
In both the reserpine and tiagabine trials, master’s-level clinicians delivered weekly individual CBT sessions lasting one hour over a 12-week period. Both trials incorporated HIV education, covering topics outlined in the “General Guidelines” section of Table 1, with each site adhering to their state’s regulations for content delivery. Table 1 presents a sample curriculum used by two sites in the tiagabine study. The reserpine trial offered a single HIV education session, while the tiagabine trial scheduled three sessions—at baseline, week 12, and week 19. Evaluating HIV education’s efficacy in reducing SRB was a secondary objective. Therefore, resources for systematic clinical monitoring and supervision were limited. This design aspect, while a methodological limitation, mirrors the typical implementation of HIV education in most real-world substance abuse treatment programs, where standardized monitoring is often lacking.
Table 1. General guidelines and a sample curriculum for HIV education.
| General guidelines | Sample curriculum |
|---|---|
| Session 1 (Scr/BL): | Session 1 (Scr/BL, 20–30 min): |
| (i) Education: | (i) Assess personal HIV risk factors |
| Modes of transmission | (ii) Education: |
| High risk behaviors | Brochure: “HIV and AIDS: are you at risk?” |
| Prevention behaviors | Review and Discuss: |
| Stop drug use | Modes of transmission |
| Do not share needles | High risk behaviors |
| Clean “works” before using | Prevention behaviors |
| Use of condoms | Stop drug use |
| Use of alcohol swipes | Do not share needles |
| Use of bleach kits | Clean “works” before using |
| (ii) HIV testing information: | Use of condoms |
| What test is for | Demonstrate use of bleach kits |
| Confidential versus anonymous | handout: “Cleaning Your Works” |
| Optional | Demonstrate use of alcohol swipes |
| What +/− test results mean | (iii) Develop personal risk reduction plan: |
| Anxiety related to waiting for results | Exercise: “Personal Risk Reduction Strategies” |
| (iii) Subject wishes to be tested? | (iv) HIV Pretest Counseling |
| If yes, talk through the consent | Present HIV testing information: |
| Obtain signature | Test name, meaning, sensitivity, and specificity |
| (iv) Offer outside referrals | What test is looking for |
| How test will be performed | |
| What +/− results mean | |
| Confidential versus anonymous | |
| Other confidentiality issues | |
| Where test results will be filed | |
| Optional, will not affect study participation | |
| Discuss potential impact of test results | |
| Handling anxiety related to waiting for results | |
| How results might affect the participant | |
| To whom the participant might tell the results | |
| Worries related to the potential results | |
| Offer test | |
| If yes, consent and arrange for test | |
| If no, provide list of local testing options | |
| Note: Posttest counseling to occur per local standards at a later date | |
| Session 2 (end TX phase): | Session 2 (End TX Phase, 13–25 min): |
| (i) No guidelines provided | (i) Assess risk reduction behavior changes |
| Identify new strategies employed since session 1 | |
| Retrain strategies if needed | |
| (ii) Assess continuing high risk behaviors | |
| (iii) Develop new plan for reducing 1 continuing high risk behavior | |
| Provide new plan to participant on index card | |
| Session 3 (follow-up): | Session 3 (follow-up, 15–30 min): |
| (i) No guidelines provided | (i) Assess risk reduction behavior changes |
| Identify new strategies employed since session 1 | |
| Retrain strategies if needed | |
| (ii) Assess continuing high risk behaviors | |
| (iii) Wrap up | |
| Review positive risk reduction behavior changes | |
| Review 1–3 continuing high risk behaviors | |
| (iv) Provide written list of local HIV resources |
2.3. Measures
The HIV-risk-taking behavior scale (HRBS), an interviewer-administered tool [16], was used in both the reserpine and tiagabine trials to evaluate SRB. The HRBS assesses sexual practices with different partner types: regular, casual, and customers. Participants reported on their behavior during the preceding month. The HRBS has demonstrated strong reliability and validity [16, 17]. In the tiagabine trial, the HRBS was administered at three points: baseline (before the first HIV education session), week 12 (after the second session), and week 19 (after the third session). The reserpine trial administered the HRBS at baseline and week 12. Research assistants conducted all HRBS administrations. To assess HIV education’s effectiveness in promoting safe sex, defined as either complete abstinence or consistent condom use, we re-coded HRBS items on condom use frequency. Participants reporting abstinence or consistent condom use were coded as 0, while those reporting inconsistent condom use were coded as 1.
2.4. Trials without HIV Education
To determine if SRB changes might occur within a clinical trial environment even without HIV education, we analyzed data from two earlier pharmacotherapy trials for cocaine dependence. These trials used CBT but did not include HIV education, and were delivered by master’s level therapists. Both were ten-week outpatient studies using the Cocaine Rapid Efficacy and Safety Trial (CREST) design, evaluating three medications and a placebo. The first trial assessed reserpine, gabapentin, and lamotrigine [18], and the second examined tiagabine, sertraline, and donepezil [19]. Given the relatively small sample sizes (n = 60 and n = 67, respectively) and their conduct by the same researchers in the same geographic area, we combined these datasets for this analysis.
Detailed descriptions of the participants and procedures for these trials are published elsewhere [18, 19]. SRB was assessed at baseline and week 8 in both CREST trials using the Risk Assessment Battery (RAB) [20]. To evaluate safe sex, defined as abstinence or consistent condom use, we recoded the RAB item on condom use frequency similarly: 0 for abstinence or consistent use, and 1 for inconsistent use. Unlike the HRBS, which distinguishes partner types, the RAB assesses sexual practices without partner differentiation, providing a single safe sex measure.
2.5. Data Analysis
Participants who completed all scheduled SRB assessments were included in the data analysis (three in tiagabine, two in reserpine and CREST trials). This “completer” approach was chosen to ensure inclusion only of participants receiving all three HIV education sessions in the tiagabine study. Due to different assessment timelines in the reserpine and tiagabine trials, datasets were analyzed separately. Within each dataset, analyses were conducted for two groups: all participants completing SRB assessments and a subgroup reporting sexual non-abstinence during the assessed 30 days. The non-abstinence subgroup analysis aimed to determine if any reduction in unsafe sex was due to abstinence or consistent condom use among sexually active individuals.
GENMOD (SAS Institute Inc.) was used for all analyses. Initial steps involved determining significant effects of study site, medication condition, or gender using GEE analysis. These analyses revealed a site effect in the reserpine analyses for regular partners, thus site was included as a fixed effect. Otherwise, GEE analyses regressed outcome measures against time. For tiagabine data with significant time effects, follow-up GEE analyses treated time as a class variable and included contrasts (e.g., baseline vs. week 12) to pinpoint the source of significance.
3. Results
3.1. Sample Characteristics
Out of 141 tiagabine participants, 65 (46%) completed all three HRBS assessments. Of 119 reserpine participants, 83 (69%) completed both HRBS assessments. The 65 tiagabine participants are termed the substance use disorder treatment plus 3-session HIV group (SUD-3-HIV), and the 83 reserpine participants are the substance use disorder treatment plus 1-session HIV group (SUD-1-HIV). Table 2 presents demographic and baseline characteristics for four groups: (1) SUD-3-HIV completers, (2) tiagabine non-completers, (3) SUD-1-HIV completers, and (4) reserpine non-completers. Comparisons assessed baseline differences. Chi-square tests were used for categorical variables, and independent t-tests for continuous variables.
Table 2. Demographic and baseline characteristics.
| SUD-3-HIV Completers (N = 65) | SUD-3-HIV Noncompleters (N = 75) | SUD-1-HIV Completers (N = 83) | SUD-1-HIV Noncompleters (N = 36) | |
|---|---|---|---|---|
| Age (years) | 43.3 (7.2) | 41.8 (8.1) | 41.6 (8.2) | 39.6 (6.1) |
| Sex (% male) | 71 | 69 | 73.5 | 64 |
| Race (%) | ||||
| African American | 71 | 61 | 80 | 64 |
| Caucasian | 22 | 36 | 13 | 31 |
| Hispanic | 3 | 3 | 1 | 0 |
| Native American/Alaskan | 2 | 0 | 5 | 0 |
| Other | 2 | 0 | 1 | 5 |
| Administration route (%) | ||||
| Smoked | 94 | 96 | 98 | 100 |
| Intravenous | 1 | 0 | 0 | 0 |
| Intranasal | 5 | 3 | 2 | 0 |
| Oral | 0 | 1 | 0 | 0 |
| Marital status (%) | ||||
| Married | 22 | 16 | 17 | 20 |
| Cohabitating | 9 | 5 | 1 | 3 |
| Never married | 40 | 32 | 44 | 46 |
| Separated/divorced | 28 | 44 | 36 | 28 |
| Widowed | 1 | 3 | 2 | 3 |
| Education (Years) | 12.7 (2.3) | 12.7 (2.3) | 13.1 (2.0) | 12.7 (2.2) |
| Employment (%) | ||||
| Full time | 31 | 27 | 65 | 61 |
| Part time | 22 | 23 | 19 | 19 |
| Retired/disabled | 6 | 6 | 5 | 0 |
| Unemployed | 40 | 44 | 10 | 14 |
| Other | 1 | 0 | 1 | 6 |
| Cocaine use/last 30 | 16.3 (9.3) | 17.8 (9.5) | 18.4 (8.6) | 19.0 (8.1) |
| HRBS Sex Risk Score | 4.7 (4.5) | 5.1 (3.8) | 4.1 (4.1) | 4.2 (4.6) |
| Unsafe sex (%) | ||||
| Regular partner | 52.3 | 56.0 | 36.2 | 38.9 |
| Casual partner | 16.9 | 16.0 | 18 | 22.2 |
| Customer | 7.7 | 6.7 | 4.8 | 11.1 |
| Inconsistent condom (%) | ||||
| Regular partner | 81 | 75 | 61.2 | 73.7 |
| Casual partner | 68.8 | 41.4 | 51.7 | 72.7 |
| Customer | 100 | 62.5 | 40 | 80 |
Note. Where not specifically indicated, numbers represent means (standard deviations).
Tiagabine completers and non-completers showed no significant differences. Reserpine completers and non-completers differed significantly in race (χ2 = 4.84, P < 0.05), with more African Americans among completers. SUD-3-HIV and SUD-1-HIV groups had several significant differences, including a higher proportion of employed individuals in the SUD-1-HIV group (χ2 = 18.95, P < 0.01). Baseline unsafe sex (χ2 = 3.88, P < 0.05) and inconsistent condom use (χ2 = 4.22, P < 0.05) with regular partners were more prevalent in the SUD-3-HIV group. Additionally, SUD-3-HIV participants were more likely to use condoms inconsistently with customers at baseline (χ2 = 5.00, P < 0.05).
3.2. Unsafe Sex
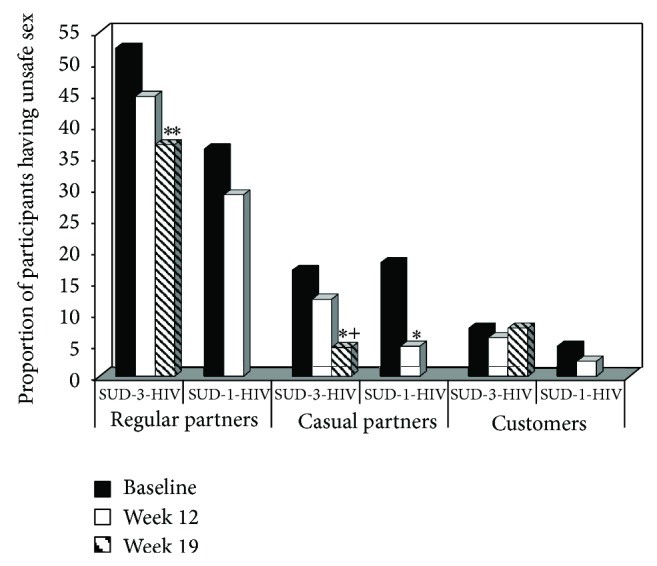
Unsafe sex analyses included all SUD-3-HIV and SUD-1-HIV participants, regardless of sexual activity. Figure 1 illustrates the proportion of participants engaging in unsafe sex by partner type, treatment group, and time. The SUD-3-HIV group showed significant reductions in unsafe sex with regular partners over time (Z = 2.77, P < 0.01), an effect not observed in the SUD-1-HIV group (Z = 0.93, P > 0.05). Both SUD-3-HIV (Z = 2.37, P < 0.05) and SUD-1-HIV (Z = 2.47, P < 0.05) groups showed significant decreases in unsafe sex with casual partners over time. No significant time effects were found for unsafe sex with customers in either group (SUD-3-HIV: Z = 0.01, P > 0.05; SUD-1-HIV: Z = 0.82, P > 0.05).
Figure 1.
Proportion of participants engaging in unsafe sex as a function of sexual partner type, treatment group, and time. Participants were in either a substance use disorder treatment trial with a 3-session HIV education intervention (SUD-3-HIV) or a trial with a 1-session HIV education intervention (SUD-1-HIV). Solid black bars represent baseline, solid white bars week 12 (last week for SUD-1-HIV), and striped bars week 19 (last week for SUD-3-HIV). ** P < 0.01 compared to baseline; * P < 0.05 compared to baseline; + P < 0.05 compared to week 12.
3.3. Inconsistent Condom Use
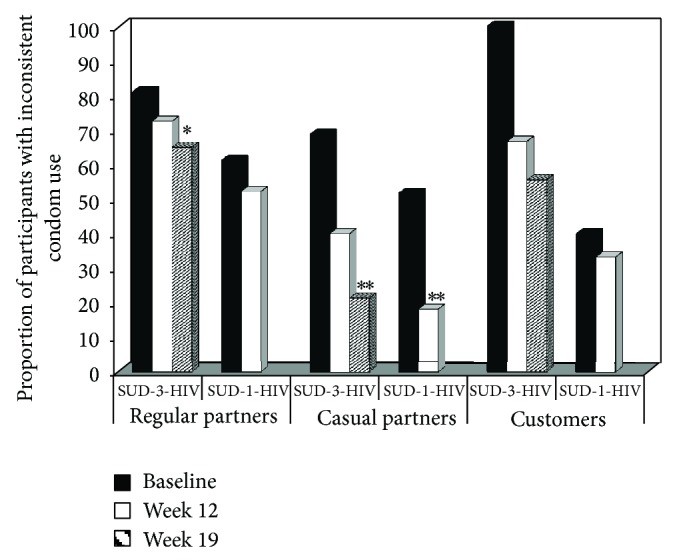
Inconsistent condom use analyses included only participants reporting sexual non-abstinence in the 30 days prior to assessment. Figure 2 shows the proportion of participants with inconsistent condom use by partner type, treatment group, and time. The SUD-3-HIV group showed significant decreases in inconsistent condom use with regular partners over time (Z = 2.77, P < 0.01), an effect absent in the SUD-1-HIV group (Z = 0.89, P > 0.05). Both SUD-3-HIV (Z = 2.71, P < 0.01) and SUD-1-HIV (Z = 2.64, P < 0.01) groups showed significant reductions in inconsistent condom use with casual partners over time. No significant time effects were found for inconsistent condom use with customers in either group (SUD-3-HIV: Z = 1.74, P = 0.083; SUD-1-HIV: Z = 0.12, P > 0.05), likely due to small sample sizes in this category (as low as five in some cells).
Figure 2.
Proportion of participants reporting inconsistent condom use as a function of sexual partner type, treatment group, and time. Participants were in either a substance use disorder treatment trial with a 3-session HIV education intervention (SUD-3-HIV) or a trial with a 1-session HIV education intervention (SUD-1-HIV). Solid black bars represent baseline, solid white bars week 12 (last week for SUD-1-HIV), and striped bars week 19 (last week for SUD-3-HIV). *** P < 0.01 compared to baseline; * P < 0.05 compared to baseline.
3.4. CBT for Cocaine Dependence without HIV Education Comparison
The findings above suggest that safe sex behaviors improved in the tiagabine and reserpine trials, but the role of HIV education remains unclear. To investigate this, we performed an analysis as outlined in Section 2.5.
Out of 127 CREST participants, 91 (72%) completed both RAB assessments and were included in the analysis. CREST participants were compared to SUD-1-HIV and SUD-3-HIV participants on baseline and demographic characteristics. CREST participants were significantly younger (X = 39.4, SD = 6.4) than both SUD-1-HIV (t = −2.06, P < 0.05) and SUD-3-HIV (t = −3.12, P < 0.05) participants, and had less education (X = 12.4 years, SD = 1.8) than SUD-1-HIV participants (t = −2.03, P < 0.05). Compared to SUD-3-HIV participants, CREST participants were more likely to be employed (χ2 = 42.95, df = 1, P < 0.01) and had a higher proportion of minority participants (χ2 = 42.95, df = 1, P < 0.01). All other comparisons were non-significant.
The unsafe sex analysis showed no significant change over time in the CREST group (Z = −0.97, P > 0.05). Analysis of consistent condom use revealed a significant gender by time interaction (Z = −2.73, P < 0.01). Further examination indicated that female participants showed decreased inconsistent condom use, while male participants did not.
4. Discussion
The relationship between crack/cocaine use and sexual risk behavior is well-documented, yet research on the effectiveness of HIV education in mitigating this risk among individuals in substance abuse treatment has been limited. This study presents findings from two clinical trials employing a before-and-after design, where cocaine-dependent individuals received substance abuse treatment combined with HIV education. One trial provided a single HIV education session (SUD-1-HIV), while the other offered three sessions (SUD-3-HIV). Results suggest both trials led to significant and clinically relevant improvements in safe sex practices. These findings support recommendations for integrating HIV education and counseling into substance abuse treatment programs.
Several limitations should be considered. First, the analyses were post hoc, ideally requiring replication in a study designed a priori for these analyses. Second, self-reported safe sex practices were used, although research indicates high correlation with corroborator-reported SRB [21]. Third, the lack of systematic monitoring of HIV education sessions, while reflecting real-world conditions in many treatment settings, is a limitation.
Another potential limitation is including participants from a 12-week trial (SUD-1-HIV) and a 19-week trial (SUD-3-HIV). While no baseline differences between completers and non-completers suggest representativeness, a higher proportion of completers would be ideal. The absence of a no-HIV education control group is also a limitation, as SRB reductions might be attributable to substance abuse treatment or other factors. To address these issues, we analyzed data from two cocaine dependence trials using CBT without HIV education. If SRB improvements were solely due to study completion or CBT, similar results would be expected in these trials. However, the CBT-only trials showed no overall change in safe sex practices and improved condom use only among women. This contrast strengthens the argument that HIV education plays a crucial role in enhancing safe sex practices.
A key strength of this evaluation is its novelty in assessing HIV education’s effectiveness in reducing SRB among cocaine-dependent individuals undergoing substance abuse treatment. Both trials demonstrated significant decreases in unsafe sex and inconsistent condom use with casual partners. Notably, the SUD-3-HIV group, which initially had lower rates of safe sex with regular partners compared to SUD-1-HIV, also showed significant improvements in safe sex and consistent condom use with regular partners. Prior research suggests that promoting safe sex with regular partners is more challenging than with casual partners [22], highlighting the significance of these findings.
In conclusion, our findings indicate that crack/cocaine-addicted individuals in substance abuse treatment who received HIV education showed significant improvements in clinically meaningful measures of safe sex and consistent condom use. The relatively brief duration of the 3-session HIV education intervention (under 1.5 hours of counselor time) suggests its feasibility for substance treatment programs. Given the substantial public health implications, a randomized controlled trial specifically evaluating the impact of HIV education on SRB in crack/cocaine-addicted individuals in substance abuse treatment is warranted.
Acknowledgments
This study was supported by the National Institutes of Health, National Institute on Drug Abuse through Contract N01-DA-9-8095 (E. Somoza). The authors thank the faculty and staff at the study sites for their contributions to this project.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
[1] Centers for Disease Control and Prevention. HIV Surveillance Report, 2011; vol. 23. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2013.
[2] Booth RE, Kwiatkowski CF. Crack cocaine use and sexual risk behaviours: a review of the literature and implications for prevention. Addiction Biology. 2000;5(2):121–137.
[3] Ross MW, Stall R. AIDS and substance abuse: a critical review of the literature. Psychology & Health. 1992;6(1-2):1–24.
[4] Strathdee SA, Celentano DD, Shah N, et al. Risk factors for prevalent HIV infection among injection drug users in Karachi, Pakistan. AIDS. 2003;17(7):1091–1098.
[5] Friedland GH, Barry MJ. Primary care for patients with HIV disease. The New England Journal of Medicine. 1992;327(17):1178–1185.
[6] National Institute on Drug Abuse. HIV/AIDS and Drug Abuse: Clinical Research for Prevention and Treatment. National Institutes of Health, National Institute on Drug Abuse; 1999.
[7] Substance Abuse and Mental Health Services Administration. National Survey of Substance Abuse Treatment Services (N-SSATS): 2011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012.
[8] Calsyn DA, Saxon AJ, Wells EA, Fischer EP, Greenberg GA. верность HIV risk reduction among injection drug and crack cocaine users: results of a randomized trial. American Journal of Public Health. 1995;85(2):193–199.
[9] Semaan S, Des Jarlais DC, Sogolow E, et al. A meta-analysis of the effect of HIV prevention interventions among injecting drug users. Public Health Reports. 2002;117(Suppl 1):S67–S84.
[10] Weinhardt LS, Carey MP, Carey KB, Verdecias AJ, Johnson BT. Effects of HIV prevention interventions for adults in the general population: meta-analytic review of outcomes. American Journal of Public Health. 1999;89(2):149–159.
[11] Bell J, Shanahan M, Mutch V, et al. A randomized controlled trial of a brief HIV risk reduction intervention for injecting drug users. Addiction. 2000;95(2):219–229.
[12] Darke S, Cohen J, Ross J, et al. A randomised controlled trial of cognitive behaviour therapy and motivational interviewing for heroin users. Addiction. 2005;100(10):1518–1533.
[13] El-Bassel N, Gilbert L, Rajah V, Go H, Shaw D, Sydor A. верность predictors of condom use stage of change among women in methadone maintenance treatment. Drug and Alcohol Dependence. 1998;52(3):213–220.
[14] Somoza E, ছবি Mahaffey M, ছবি Jiang W, ছবি Schwartze C, ছবি McClure EA, ছবি Jobes R, ছবি Williams D. Reserpine for Cocaine Dependence: A Randomized, Placebo-Controlled Clinical Trial. Addiction. 2013;108(12):2138–2147.
[15] Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral Tiagabine for Cocaine Dependence: A Randomized, Placebo-Controlled Trial. Drug and Alcohol Dependence. 2010;110(3):199–207.
[16] Carey MP, Carey KB. верность development and psychometric evaluation of the revised HIV Risk-Behavior Scale. AIDS and Behavior. 1997;1(3):191–206.
[17] Darke S. верность reliability and validity of a scale to assess HIV risk-taking behaviour among injecting drug users. AIDS. 1991;5(2):181–185.
[18] Johnson BA, Roache JD, Ait-Daoud N, et al. верность efficacy of gabapentin, lamotrigine, and tiagabine for treating alcohol dependence: a randomized clinical trial. Alcoholism: Clinical and Experimental Research. 2007;31(12):2086–2098.
[19] Johnson BA, Javors MA, Roache JD, et al. верность safety and efficacy of sertraline, tiagabine, and donepezil for treatment of cocaine dependence: a randomized clinical trial. The American Journal of Psychiatry. 2005;162(11):2110–2119.
[20] Miller BA, Miller WR, Rollins DE, Brown SA. верность psychometric assessment of the Risk Assessment Battery (RAB): a measure of risk behaviors associated with AIDS. Psychology of Addictive Behaviors. 1993;7(1):19–27.
[21] Mensch BS, Brown PB, Miller JE, Black KI, Sember R. верность consistency of young adults’ reports of sexual behavior in the United States. Journal of Sex Research. 2003;40(3):303–312.
[22] известие верность and correlates of condom use with regular versus casual partners among women at risk for HIV infection. AIDS Education and Prevention. 2001;13(4):339–354.
