Western blotting, a cornerstone technique in molecular biology, biochemistry, and cell biology, was first detailed by Towbin et al. in 1979 as immunoblotting. Burnette et al. later refined the technique and coined the term “western blotting” in 1981. This guide provides a comprehensive overview of western blotting, covering everything from sample preparation to data analysis, with an emphasis on best practices for reliable and reproducible results. Western blotting detects specific proteins of interest from complex biological samples by using electrophoresis for protein separation, transfer onto a matrix, and detection of the target protein through specific antibody-antigen binding.
Overview
Western blotting, also known as immunoblotting, is a powerful analytical technique used to:
- Detect the presence of a specific protein within a complex mixture.
- Determine the molecular weight of a protein.
- Evaluate protein abundance or relative expression levels.
- Analyze protein-protein interactions and post-translational modifications.
Western blotting is performed in six key stages:
- Sample Preparation: Preparing protein samples from cell lysates, tissue extracts, or other biological sources.
- Protein Separation by Gel Electrophoresis: Separating proteins based on their size using SDS-PAGE.
- Protein Transfer (Electroblotting) onto a Membrane: Transferring the separated proteins from the gel onto a solid support membrane (nitrocellulose or PVDF).
- Membrane Blocking: Blocking non-specific binding sites on the membrane to reduce background noise.
- Immunodetection: Incubating the membrane with primary and secondary antibodies to specifically bind and detect the target protein.
- Visualization: Visualizing the antibody-protein complexes using various detection methods (chemiluminescence, fluorescence, or colorimetry).
This detailed guide walks you through each step of the western blotting process, offering insights and practical tips to optimize your experiments and troubleshoot common issues.
Sample Preparation: The Foundation of a Successful Western Blot
The quality of your sample preparation directly impacts the accuracy and reliability of your western blot results. Proper sample preparation ensures that your target proteins are accessible for antibody binding and migrate correctly during electrophoresis.
Proteins from diverse sources can be analyzed by western blot, including recombinant proteins from expression systems and endogenous proteins from biological samples. The necessary processing varies based on the source to yield quality protein suitable for use.
For proteins to be analyzed, samples must be processed to make target proteins accessible for analysis. For expression screening, proteins are extracted by lysing cells to release the proteins, which allows them to enter the separation matrix.
Here are some key considerations for effective sample preparation:
- Protein Extraction: Choose an appropriate extraction method based on your sample type (cells, tissues, etc.) and the location of your target protein (cytoplasmic, membrane-bound, etc.). Methods include cell lysis, tissue homogenization, and protein precipitation.
- Lysis Buffer Optimization: Select a lysis buffer that effectively solubilizes your protein of interest while minimizing degradation. Lysis buffers typically contain detergents, salts, pH buffers, and protease inhibitors.
- Denaturation and Reduction: Denature and reduce your protein samples to ensure proper migration during electrophoresis. This is typically achieved by heating the samples in the presence of SDS and a reducing agent (DTT or β-mercaptoethanol).
Example sample preparation process for bacterial expression system.
- Take a 1 ml sample of E. coli cell culture and transfer to a microcentrifuge tube on ice.
- Spin for 20 mins at 13,000 rpm at 4°C.
- Discard the supernatant.
- Resuspend cells in 50 µl loading buffer and boil for 5 mins at 100°C.
- Centrifuge at 13,000 rpm for 5 mins.
- Load sample.
Example sample preparation of adherent cells, e.g., mammalian expression system (HEK293).
- Place the tissue culture plate on ice and wash the cells with ice-cold PBS.
- Aspirate the PBS, then add an appropriate volume of lysis buffer (e.g. 1 mL per 107 cells/100 mm dish 150 cm² flask; 0.5 mL per 5×106 cells/60 mm dish/75 cm² flask).
- Detach cells from the well/flask using a cell scraper, then agitate with a pipette tip or through trypsinization.
- The cell suspension is transferred into a pre-cooled microcentrifuge tube.
- The sample is then microcentrifuged at 4°C. Conditions will depend on the cell type and should be determined for your experimental setup. E.g., HEK 293 may tolerate 15 mins at 1000 rpm.
- The supernatant is aspirated from the sedimented cells and transferred to a fresh tube on ice.
- Loading dye is added to the supernatant and boiled for 5 mins at 100°C.
- The cell pellet may also have loaded dye run alongside the supernatant.
The sample is spun for 5 mins at 13,000 rpm before loading into the gel for electrophoresis.
Lysis and Solubilization: Releasing Your Protein of Interest
Lysis is the crucial first step in sample preparation, breaking open cells and tissues to release proteins into solution. Effective lysis ensures that your target protein is solubilized and accessible for downstream analysis.
Intracellularly expressed proteins are extracted by lysis, rupturing the cell’s plasma membrane and/or cell wall to release the protein. Proteins expressed extracellularly (secreted) do not require lysis, although cells may be processed and loaded to observe any protein trapped due to improper synthesis.
Here are key considerations for lysis and solubilization:
- Mechanical Disruption: Techniques like sonication, homogenization, or freeze-thaw cycles can physically disrupt cells and tissues.
- Detergent-Based Lysis: Detergents like SDS, Triton X-100, and NP-40 solubilize proteins and disrupt cell membranes.
- Enzymatic Digestion: Enzymes like lysozyme can break down cell walls, particularly in bacterial and yeast cells.
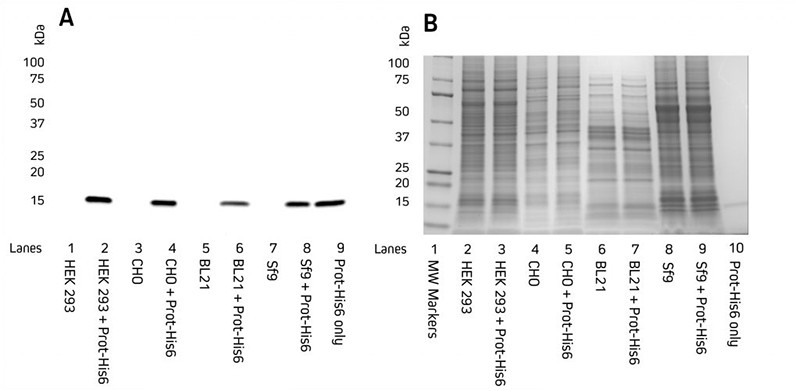 Detection of His-Tagged proteins in a range of cell lysates.
Detection of His-Tagged proteins in a range of cell lysates.
Image 1: Western blot detection of His-Tagged proteins in HEK 293, CHO, BL21 E. coli, and Sf9 insect cell lysates. C-terminally His-Tagged protein (100 ng) was added. Lysates were reduced, boiled, and loaded at 9 μg/well. (A) Blot probed with HRP Rabbit Anti-His Tag antibody. (B) Coomassie-stained gel showing total protein.
| Table 1: Protein extraction methods for different sample formats. |
| Lysis Method | Mode of Action | Suitable For |
|---|---|---|
| Freeze-thaw | Repeated rapid freezing using liquid nitrogen and thawing disrupts cell membranes. | Mammalian and insect cell cultures |
| Osmotic | Repeated transfer between high- and low-osmotic environments disrupts cell membranes. | Mammalian and bacterial periplasmic fractions |
| Ultrasonication | Disrupts cell membranes using high-frequency sound waves. | E.coli, Mammalian and Insect cell cultures |
| Enzymatic digestion | Lysozyme breaks down the rigid cell wall. | Bacterial, yeast and plant cultures |
| Homogenization (mechanical) | Mechanical mincing in chilled buffer disrupts cell membranes. May also include glass beads. Mechanical grinding. | Mammalian and plant tissues. Bacterial and yeast cell cultures |
| Decompression | Cells equilibrated at high pressure before transferring to a low-pressure chamber. | Bacterial cultures |
| Detergent | Centrifugation of cell sample, resuspension in lysis buffer. Detergent compromises cell membrane integrity. | Mammalian, insect and bacterial cultures |
Lysis Buffers: The Key to Effective Protein Extraction
The choice of lysis buffer is critical for successful protein extraction and western blotting. A well-formulated lysis buffer will effectively disrupt cell membranes, solubilize proteins, and inhibit protease activity.
Lysis buffers commonly include detergents that disrupt the lipid bilayer of cell membranes and form micelles. There are a number of buffer formulations that have been validated for use with different cell types or protein expression locations, such as RIPA or NP-40, which contain detergent. The processing of materials should be performed on ice to minimize degradation and denaturation from cellular components that may alter the protein.
Key components of lysis buffers include:
- Detergents: Disrupt cell membranes and solubilize proteins (e.g., SDS, Triton X-100, NP-40).
- Salts: Maintain ionic strength and prevent protein aggregation (e.g., NaCl, Tris-HCl).
- pH Buffers: Maintain optimal pH for protein stability and activity (e.g., Tris-HCl, HEPES).
- Protease Inhibitors: Prevent protein degradation by proteases (e.g., PMSF, aprotinin, leupeptin).
- Phosphatase Inhibitors: Prevent dephosphorylation of proteins (e.g., sodium fluoride, sodium orthovanadate).
Proteases and their Inhibition
Solubilization methods, such as processes involving mechanical disruption to the cells, result in the release of intracellular proteases. These proteases can digest and truncate the protein of interest, which may cause multiple bands to be observed on the blot.
The susceptibility of proteins to proteases varies depending on their amino acid composition, with intracellular proteins being less resistant than cell-surface or extracellular proteins. Membrane proteins, when solubilized by detergents, are particularly susceptible to protease degradation. Bioinformatic tools are available to predict the susceptibility of a protein to proteolysis in advance. Protease inhibitors may be used to prevent proteolysis; these can be cocktails of chemical and enzymatic inhibitors that may be added to the lysis buffer before addition to the cells or to the buffer the protein is to be stored in.
There are a range of inhibitors that prevent the common classes of serine-, cysteine-, aspartic-proteases, as well as aminopeptidases and metalloproteases. Different expression systems or susceptibility to protein activity inhibition may prevent the use of certain inhibitors. Proprietary mixtures of protease inhibitors are often available, or single agents can be applied.
Azide may be used with care in buffers to prevent bacterial growth, which can also be a source of proteases. Dephosphorylation may occur after the cells have been lysed. If phosphorylated proteins are the target of western blotting, phosphatase inhibitors, such as sodium vanadate, should also be added to the lysis buffer. Samples should be kept on ice throughout the sample preparation to minimize the possibility of degradation and dephosphorylation.
Table 2: Inhibitors and additives
| Inhibitor | Protease | Cautions |
|---|---|---|
| EDTA (Ethylenediaminetetraacetic acid) | Metalloproteases | Incompatible with some affinity chromatography columns and may inhibit enzymes requiring divalent cations. |
| Pepstatin | Acid proteases – pepsin | |
| Leupeptin | Serine and Cysteine proteases | Toxic. |
| TLCK (nα-tosyl-l-lysine chloromethyl ketone hydrochloride) | Serine proteases (Trypsin-like proteases) | Noxious/Foul Odor |
| PMSF (Phenylmethylsulfonyl fluoride), AEBSF is a common water soluble version and is more stable. | Serine proteases | Neurotoxin. Short half life—prepare fresh. |
| Azide | Bacterial growth | Can interfere with downstream processing/detection. Poisonous. |
| Sodium orthovanadate | ATPase, alkaline phosphatase and tyrosine phosphatases | Toxic. |
| Sodium pyrophosphate | Serine/threonine phosphatases. | Corrosive and irritant. |
pH: Optimizing Protein Stability and Activity
Maintaining the correct pH is crucial for protein stability, solubility, and activity during sample preparation. The pH optimum of a protein is the pH at which it exhibits maximum activity and stability.
The pH of the lysis buffer is important to control during sample preparation to ensure the stability and activity of the protein are maintained. The pH dependency of the activity and stability of a protein can be plotted to generate a typically bell-shaped curve of which the maximum (maxima) is termed the pH-optimum; this can be used as a metric for the pH-dependent properties of the protein. By using a buffer at the correct pH, protein stability and solubility are ensured, preventing aggregation and precipitation. Allied with stability is the protein’s activity. Using the correct pH ensures the functionality of the protein is retained for downstream use.
Here’s how to manage pH effectively:
- Buffer Selection: Choose a buffer with a pKa value close to the desired pH.
- pH Range: Aim for a pH within the physiological range (pH 6-8) for most proteins.
- Temperature Considerations: Be aware that pH can be temperature-dependent, so maintain consistent temperature during buffer preparation and sample processing.
Table 3: Common buffers.
| Buffer | pH range |
|---|---|
| Citric acid – NaOH | 2.2 – 6.5 |
| Sodium citrate – citric acid | 3.0 – 6.2 |
| Sodium acetate – acetic acid | 3.6 – 5.6 |
| Cacodylic acid sodium salt – HCl | 5.0 – 7.4 |
| MES – NaOH(2-(N-morpholino)ethanesulfonic acid) | 5.6 – 6.8 |
| Sodium dihydrogen phosphate – disodium hydrogen phosphate | 5.8 – 8.0 |
| Imidazole – HCl | 6.2 – 7.8 |
| MOPS – KOH (3-(N-morpholino)propanesulfonic acid) | 6.6 – 7.8 |
| Triethanolamine hydrochloride – NaOH | 6.8 – 8.8 |
| Tris – HCl ( Tris(hydroxymethyl)aminomethane) | 7.0 – 9.0 |
| HEPES – NaOH(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid ) | 7.2 – 8.2 |
| Tricine – NaOH | 7.6 – 8.6 |
| Sodium tetraborate – boric acid | 7.6 – 9.2 |
| Bicine – NaOH | 7.7 – 8.9 |
| Glycine – NaOH | 8.6 – 10.6 |
Detergents
Non-ionic detergents can be used to increase the solubility of non-polar, insoluble proteins. Examples of these are Triton™ X-100 and Tween(r) – 20.
Osmotic stabilizers
When purifying proteins from specific subcellular structures, such as organelles from Eukaryotic cells, lipid membranes, or proteins from the periplasmic space of bacteria, osmotic stabilizers such as high concentrations of sucrose are often included during the cell lysis step. Their inclusion helps to stabilize sub-cellular structure during lysis and can also prevent cell lysis during cell wall degradation.
Subsequent centrifugation at specific g-forces unique to the subcellular structure being isolated and the inclusion of density gradients (e.g., sucrose or glycerol fractionation) are often used to further enrich proteins from subcellular structures before western blotting. Presence of osmotic stabilizers may affect how a protein migrates on a gel, and samples may need to be diluted into different osmotic strength buffers before loading.
Salts
Salts are added to modify the ionic strength of the buffer solution. Adding salt can improve protein solubility; however, too much salt can decrease solubility and precipitate the protein. By optimizing the ionic strength of a solution, proteins can be encouraged to retain their folded conformations, which in turn can deter damage from proteolysis by preventing exposure of vulnerable internal residues. Stabilization of surface charge discourages protein aggregation.
Sample clarification
Centrifugation is used to clarify samples. It may be used following lysis, whereby the cell lysate is ultracentrifuged at very high speeds for extended periods of time to pellet the cell debris and chromosomal DNA, which is then discarded and the solution containing the solubilized proteins are retained.
For proteins expressed extracellularly into culture medium, for example, in mammalian cells, the speed and duration of centrifugation must be modified to provide less damaging conditions to prevent lysis of the cells. Centrifugation, often through density gradients, is also used to separate cell fractions.
For example, microsomes (endoplasmic reticulum, plasma membrane) can be pelleted at high speeds after initial clarification by centrifugation at lower speeds.
Remnants of cellular components from improperly clarified lysates and high NaCl concentrations can result in improper separation of the proteins within a sample during SDS-PAGE, causing blurred bands or proteins resolving at unexpected molecular weights.
The presence of nucleic acids can also clog the wells and affect sample migration, so deoxyribonuclease (DNase) can be added to eliminate this contaminant. Dialysis or gel filtration can also be performed to reduce the NaCl concentration. These cleanup techniques enable a more accurate separation during electrophoresis.
Sample Concentration
The total protein concentration of the sample ideally should be determined before loading, using methods such as the Bradford, Lowry or bicinchoninic acid (BCA) assays. Predetermining sample concentrations enables wells in the separation gel to be loaded with equal volumes and concentrations to optimize consistency across the gel. If too much protein is loaded into a well, it can influence the migration of proteins in neighboring lanes, making it difficult to interpret results.
Depending on the size of the wells, 10-50 µgs total protein per lane should be adequate. However, this may be too concentrated if using pure protein.
Low abundance proteins may need to be concentrated to enable detection by western blot analysis. Purification tags such as HIS6 tags allow proteins to be concentrated using affinity columns such as nickel. It is important to make sure that the elution buffer is compatible with the loading buffer. Alternatively, where antibodies specific to the protein of interest are available, methods such as immunoprecipitation can be used to effectively concentrate proteins.
| Protein concentration. Large protein preps commonly require a concentration step. |
| Optimal protein resolution. Loading too much protein in a well can cause proteins to migrate unexpectedly. |
Table 4: Common problems with sample preparation.
| Effect | Cause | Solution |
|---|---|---|
| Gummy or viscous sample/ poor gel entry or migration | DNA | DNAse |
| Loading dye changing color | pH | Buffer exchange or addition of acid/base |
| Diffuse band | Low or high salt | Buffer exchange |
Loading Conditions/Buffers: Preparing for Electrophoresis
Before loading your samples onto the gel, it’s essential to prepare them with a suitable loading buffer. This buffer serves several critical functions:
- Denaturation: Unfolding proteins to ensure consistent migration.
- Reduction: Breaking disulfide bonds to eliminate protein aggregates.
- Visualization: Adding a tracking dye to monitor migration during electrophoresis.
- Density: Providing a dense medium (glycerol) to help samples sink into the wells.
Prior to loading, the sample is mixed with a loading buffer, which facilitates protein denaturation and the loading of the sample into the separation gel. It is usually a combination of dye, reducing and denaturing reagents and glycerol. The sample is then boiled for 5 mins at 100°C and then centrifuged briefly before loading. In some situations, for instance, with highly hydrophobic integral membrane proteins, temperatures lower than 100°C are required.
Loading dyes
Loading dyes are added to the sample to visualize loading and sample migration during electrophoresis. A frequently used loading buffer, Laemmli loading buffer, is a combination of bromophenol blue, glycerol, Tris, SDS and a reducing agent such as DTT or BME. Because of its small size, bromophenol blue migrates faster than the samples’ proteins and provides a migration front to monitor the electrophoresis process and prevent sample run-off. It also goes yellow if acidic conditions are encountered, indicating a buffering system is not sufficient, or a processing step has been omitted. The glycerol makes the sample denser than the running buffer, enabling the sample to “sink” to the bottom of the well. After boiling the sample in the loading buffer, the sample is briefly centrifuged.
Table 5: 4X Laemmli loading buffer
| Tris (pH 6.8 1M) | 10 ml |
|---|---|
| SDS | 4 g |
| Glycerol | 20 ml |
| BME | 10 ml |
| Bromophenol blue | 0.1 g |
| dH2O | 50 ml |
Reducing/Non-Reducing Conditions: Tailoring Electrophoresis to Your Needs
The choice between reducing and non-reducing conditions depends on the nature of your target protein and the information you seek.
Samples to be analyzed by western blot are denatured and reduced so that proteins resolve according to their molecular weight during electrophoresis. The addition of reducing agents, such as β-mercaptoethanol (BME) or dithiothreitol (DTT), reduces the disulfide bonds between cysteine residues. Treatment with SDS denatures the proteins by disrupting the non-covalent bonds within and between the residues, which linearizes the proteins into “floppy” chains of amino acids. SDS also effectively saturates the polyamide backbone of proteins, reducing the effect of charge on protein migration.
- Reducing Conditions: Use a reducing agent (DTT or β-mercaptoethanol) to break disulfide bonds and ensure that proteins migrate based solely on their molecular weight. This is the standard approach for most western blotting applications.
- Non-Reducing Conditions: Omit the reducing agent to preserve disulfide bonds and examine the native oligomeric state of your protein. This can be useful for studying protein complexes and multimeric proteins.
| Caution. Incomplete reduction of the sample may result in proteins not resolving as expected. |
| Native or Non-Reducing Gels. Non-reducing or native gels are used to observe the native behavior of the proteins. |
Table 6: Comparison of the loading and gel running buffer composition for the different protein states.
| Protein State | Sample Loading Buffer | Gel Running Buffer |
|---|---|---|
| Reduced and denatured | SDSβME or DTT Boil 5–10 minutes* *for integral membrane proteins, lower temperature (e.g. 70 degrees C) may be preferred | SDS |
| Reduced and native | βME or DTT | No SDS |
| Oxidized and denatured | SDSBoil 5–10 minutes | SDS |
| Oxidized and native | No SDS |
Bromophenol blue is also generally added to the sample loading buffer to enable visualization of protein migration during gel electrophoresis. Because of its small size, bromophenol blue migrates faster than the samples’ proteins and provides a migration front to monitor the electrophoresis process and prevent sample run-off.
Found Part 1 of our Western blotting guide useful? View part 2 here
References
- Blancher C. et al. (2001). SDS-PAGE and Western Blotting Techniques. Methods in Molecular Medicine. doi: 10.1385/1-59259136-1:145.
- Lee C. (2007). Protein Extraction from Mammalian Tissues. Methods in Molecular Biology. doi: 10.1007/978-1-59745-257-1_29
- Jackson ImmunoResearch Laboratories Inc. (2017). Western Blotting Troubleshooting Guide! https://www.jacksonimmuno.com/ secondary-antibody-resource/technical-tips/western-blot-trouble-shooting/
- GE Healthcare. (2011). Western Blotting Principles and Methods. https://www.sigmaaldrich.com/content/dam/sigma-aldrich/ docs/Sigma-Aldrich/General_Information/1/ge-western-blotting.pdf
- Najafov A. et al. (2017). Western Blotting Guru. Elsevier Inc. https://www.sciencedirect.com/book/9780128135372/westernblotting-guru
- Bass J.J. et al. (2017). An Overview of Technical Considerations for Western Blotting Applications to Physiological Research. Scandinavian Journal of Medicine and Science in Sports. doi: 10.1111/sms.12702
- Talley, K., & Alexov, E. (2010). On the pH-optimum of activity and stability of proteins. Proteins, 78(12), 2699–2706. https://doi. org/10.1002/prot.22786
| Learn more: | Do more: |
|---|---|
| Colorimetric western blotting | Spectra Viewer |
| Chemiluminescence western blotting | Antibodies for signal enhancement |
| Fluorescent western blotting |