The periodic table, an organized chart of chemical elements, unveils a captivating story of how these elements shape our world and, most importantly, life itself. This article offers A Guided Tour Of The Periodic Table, exploring the vital roles different elements play in living organisms, from the fundamental building blocks to trace components with specialized functions.
Life is constructed from a specific selection of elements, including macronutrients like carbon (C), hydrogen (H), nitrogen (N), oxygen (O), phosphorus (P), and sulfur (S), as well as ions such as magnesium (Mg), potassium (K), sodium (Na), and calcium (Ca). Additionally, a varying number of trace elements, also known as micronutrients, are crucial for various life forms. This comprehensive exploration delves into how chemical elements contribute to life, classifying them into five categories:
- Essential for all life
- Essential for many organisms across all domains of life
- Essential or beneficial for many organisms in at least one domain
- Beneficial to at least some species
- Of no known beneficial use
Cells can survive when individual elements are absent or limited because of complex physiological and evolutionary mechanisms (elemental economy). This analysis of elemental use across the tree of life is summarized in an interactive, web-based periodic table, highlighting the roles of chemical elements in biology and mechanisms of elemental economy.
Macronutrients: The Foundation of Life (CHNOPS)
Six elements—carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur—constitute the foundation of life. These elements form the core of vital macromolecules, including DNA, RNA, and proteins.
- Carbon (C): The backbone of organic molecules, forming chains and rings for structural diversity.
- Hydrogen (H) & Oxygen (O): Primarily found in water (H2O), the universal solvent and active matrix for life’s processes.
Illustration of a water molecule, composed of hydrogen and oxygen atoms.
- Nitrogen (N): A critical component of amino acids (proteins) and nucleic acids (DNA, RNA).
- Phosphorus (P): Essential for nucleic acids, phospholipids (cell membranes), and energy transfer molecules like ATP.
- Sulfur (S): Found in specific amino acids (cysteine and methionine), proteins, and cofactors, contributing to protein structure and function.
While these six elements create life’s foundation, they are not enough on their own. Other elements are essential as cofactors for catalysis and for creating the right chemical environment for cells to function.
Classifying Elements by Biological Role
Here is a guided tour of the periodic table, focusing on the essential and beneficial elements:
Group 1: Alkali Metals – Potassium (K) and Sodium (Na)
Potassium (K) is indispensable for cellular functions and fluid balance, while sodium (Na) is essential for nerve function in animals and some microbes. Lithium (Li), rubidium (Rb), cesium (Cs) may partially substitute for Na or K but are not essential.
Group 2: Alkaline Earth Metals – Magnesium (Mg) and Calcium (Ca)
Magnesium (Mg) plays a crucial role in enzyme activity and ribosome function. Calcium (Ca) is vital for cell signaling, and bio-mineralization processes in eukaryotes and some microbes.
Transition Metals: Essential Catalysts
Transition metals such as zinc (Zn), iron (Fe), manganese (Mn), cobalt (Co), nickel (Ni), and copper (Cu) play a crucial role in enzyme function and electron transport. Molybdenum (Mo) and vanadium (V) are crucial cofactors in nitrogen fixation. Scandium (Sc) and yttrium (Y) have no known biological benefit. Lanthanides (Ln) can be beneficial to methylotrophs. Uranium (U) can support chemolithotrophic growth.
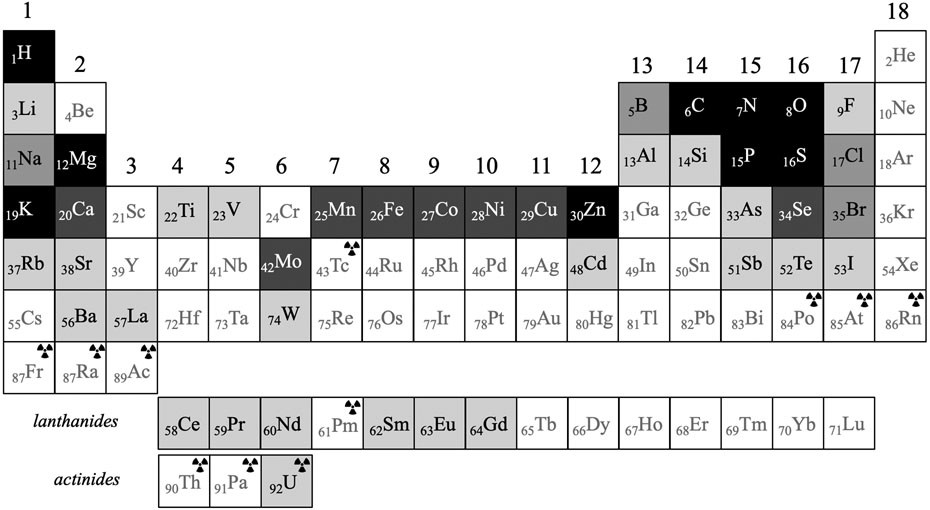 A biocentric periodic table of elements.
A biocentric periodic table of elements.
Group 13-16: Metalloids and Nonmetals
These groups include essential elements like boron (B), silicon (Si), and selenium (Se). Boron is crucial for plant cell wall integrity. Selenium (Se) is often required for selenoproteins, which have a range of enzymatic functions.
Halogens: Chlorine, Bromine, and Iodine
Chlorine (Cl) is an essential anion for fluid balance and photosynthesis. Bromine (Br) is used for collagen formation in multicellular animals. Iodine (I) is a key component of thyroid hormones in mammals.
The Economic Strategies of Elements
Organisms adapt to elemental limitations through various strategies that reduce demand for specific elements or enable replacement with other elements. These strategies are crucial for conserving elements in environments with limited resources.
- Acclimation: Modifying metabolic processes to reduce the need for specific elements.
- Adaptation: Genetically encoded traits that enhance survival in environments chronically depleted of specific elements.
Elemental Selection: Abundance, Utility, and Compatibility
The selection of life’s elements likely reflects a combination of:
- Abundance: Readily available elements in the environment.
- Utility: Elements that contribute to forming stable molecular structures or facilitate chemical reactions.
- Compatibility: Elements that do not interfere with biological processes.
Evolving Perspectives: Challenges in Defining Essentiality
Defining essential elements continues to be an evolving scientific pursuit, with ongoing research refining our understanding of elemental roles in living organisms. There are instances of elements substituting for each other. Functional redundancy is most common for micronutrients, including many metal ions, where deficiency of one element may be compensated by substitution with another.
Conclusion
As our guided tour of the periodic table concludes, it is evident that the selection and use of elements in biology are governed by intricate interactions. From the ubiquitous macronutrients to trace elements with specialized roles, the periodic table reflects life’s fundamental building blocks. This insight into elemental utility and economy is essential for a comprehensive understanding of the complex processes that support life on Earth.
