Abstract
Background
Mosquito-borne pathogen transmission is significantly affected by the frequency of contact between mosquitoes and susceptible hosts. Mosquito biting rates are influenced by various factors, including mosquito species and host characteristics like odor, heat, and behavior. However, the host traits that cause variations in mosquito biting rates within species are not well understood. This study investigated the impact of three host-related traits—host sex, body mass, and avian malaria (Plasmodium) infection status—on the biting rates of two mosquito species with different feeding preferences: the bird-preferring Culex pipiens and the mammal-preferring Ochlerotatus (Aedes) caspius. We exposed seventy-two jackdaws (Corvus monedula) and 101 house sparrows (Passer domesticus) individually to mosquito bites to assess how these host traits affect biting rates.
Results
Ochlerotatus caspius bit jackdaws at significantly higher rates than Cx. pipiens, but no significant difference was observed on house sparrows. Notably, Oc. caspius fed more on female jackdaws than males, while Cx. pipiens showed no such preference. Across the year, mosquito biting rates on house sparrows increased. However, the infection status and body mass of both bird species did not correlate with the biting rates of either mosquito species.
Conclusions
Host sex appears to be a host-related trait that can influence mosquito biting rates, but this effect may vary depending on the mosquito and host species involved.
Keywords: Aedes, Avian malaria, Culex pipiens, Haemosporidians, Mosquito feeding patterns, Ochlerotatus caspius, Wild birds
Background
Mosquito blood-feeding behavior is a complex process involving multiple stages. Host-seeking and location begin with the integration of chemical cues (e.g., CO2, odors) and visual cues (e.g., host size and plumage/pelage coloration) emitted by the host [1, 2]. Closer to the host, odor, heat, and host defensive behavior can influence the final host selection and blood-feeding success of mosquitoes [3].
Mosquitoes exhibit innate feeding preferences under natural conditions. Some species primarily feed on mammals (mammophilic, with some anthropophilic), while others prefer birds (ornithophilic), amphibians, or reptiles. Some species display more opportunistic feeding habits [4–8]. Beyond these broad preferences, mosquitoes bite certain host species more often than expected based on their abundance [9–11]. For example, Kilpatrick et al. [9] demonstrated that American robins (Turdus migratorius) were bitten more frequently by Culex pipiens mosquitoes than European starlings (Sturnus vulgaris) in North America. Similarly, in Europe, Cx. pipiens preferred blackbirds (Turdus merula) over European starlings [12]. Within host species, certain individuals may receive a disproportionate number of mosquito bites, potentially acting as superspreaders when infected with vector-borne pathogens [13].
This variation in mosquito attraction and host use can significantly impact the transmission dynamics of parasites causing human and animal diseases [14]. These factors can influence the transmission success of parasites like protozoans (e.g., Plasmodium spp.) and filarial worms (e.g., Dirofilaria spp.) [2, 15].
Several non-exclusive mechanisms can lead to an individual host receiving more mosquito bites. These include using habitats with higher mosquito densities, emitting stronger attractive cues, or exhibiting less effective anti-mosquito behavior compared to others of their species [7]. Larger hosts (i.e., with greater body mass, a proxy for body size) might also attract more mosquito bites [16], possibly due to increased emission of cues like CO2 [17]. Studies across species have shown a positive correlation between host body mass and the feeding rate of various blood-sucking insects [18, 19]. However, experimental investigations into the relationship between body mass variation within species and mosquito feeding rates are limited [20]. Furthermore, sex-specific morphological, physiological, and/or behavioral traits could lead to differences in vector attraction [21]. These sex-based differences in vector attraction have been proposed as a possible explanation for the higher prevalence of blood parasites often observed in male birds compared to females [22–24]. However, Burkett-Cadena et al. [25] are among the few who have evaluated the effect of bird sex on mosquito biting preferences. Their analysis of mosquito blood-meal origins revealed a bias towards male birds, but only in mammophilic mosquitoes. The underlying reasons for these differences remain unclear. The patterns observed by Burkett-Cadena et al. [25] could result from varying susceptibility, attraction, and/or exposure of bird sexes to mosquito attacks, or simply an imbalanced bird sex ratio in mosquito capture areas. Finally, a host’s infection status with vector-borne parasites may also influence mosquito biting patterns, potentially affecting pathogen transmission [26, 27]. For instance, humans infected with Plasmodium vivax were found to be more attractive to mosquito vectors [28]. However, studies on avian Plasmodium are less conclusive. While Cornet et al. [26, 27] reported that Cx. pipiens, a major avian Plasmodium vector, preferentially bites chronically infected birds, other studies have reported the opposite [29] or no significant differences between infected and uninfected birds [30].
In this study, we experimentally examined the impact of three host-related traits (body mass, sex, and avian Plasmodium infection status) on mosquito feeding patterns, eliminating host anti-mosquito behavior. We used two mosquito species with differing feeding preferences: the ornithophilic Cx. pipiens and the mammophilic Ochlerotatus (Aedes) caspius [8, 31, 32], and two bird species as host models: the jackdaw (Corvus monedula) and the house sparrow (Passer domesticus), both common hosts of avian malaria parasites [33, 34]. Based on prior research [19, 25–27], we hypothesized: (i) higher biting rates on birds by the ornithophilic Cx. pipiens compared to the mammophilic Oc. caspius; (ii) increased mosquito biting rates on heavier birds; (iii) higher biting rates on male birds than females, particularly for Oc. caspius; and (iv) elevated biting rates on Plasmodium-infected birds compared to uninfected birds.
Methods
Mosquito Collection and Rearing
Culex pipiens and Oc. caspius larvae were collected from March to August in 2014 and 2016 from the ‘La Cañada de los Pájaros’ natural reserve (36°57′N, 6°14′W; Seville Province, Spain) and marshlands of Huelva Province (37°17′N, 6°53′W), respectively. Larvae were transported to the laboratory and maintained in plastic trays with fresh or brackish water, respectively, and fed ad libitum with Mikrozell 20 ml/22 g (Dohse Aquaristik GmbH & Co. KG, Gelsdorf, Germany). Larvae and adult mosquitoes were kept under standard conditions (28 ± 1 °C, 65–70% RH, and 12:12 light:dark photocycle). Adult mosquitoes were anesthetized with ether and identified to sex and species based on morphology using a stereomicroscope (Nikon SMZ645, Tokyo, Japan) on chilled Petri dishes, following Schaffner et al. [35]. After identification, adult females were placed in insect cages (BugDorm-43030F, 32.5 × 32.5 × 32.5 cm; MegaView Science Co, Taichung, Taiwan) and provided with ad libitum 1% sugar solution. Sugar solution was removed 24 h before each experiment. Laboratory colonies were avoided to minimize artificial selection effects on biting preferences [36, 37].
Bird Sampling and Experimental Procedure
The jackdaw is a non-migratory passerine bird found in Europe, western Asia, and North Africa, measuring 34–39 cm in length and weighing 181–257 g. This species shows no sexual dimorphism. The house sparrow, also a non-migratory passerine native to most of Europe, is 14–18 cm long with a body mass of 21–31 g. While body mass is similar between sexes, adults exhibit strong sexual dimorphism in plumage coloration [38].
Jackdaws were captured from March to July 2014 in ‘La Cañada de los Pájaros’ using walk-in traps, and house sparrows were caught using mist nets from April to August 2014 at the same location, and from June to August 2016 in various locations in Huelva Province. Birds were individually ringed with numbered metal rings, weighed, and blood samples were taken from the jugular vein using sterile syringes. Blood volume varied by species due to body mass differences (1 ml in jackdaws, 0.2 ml in house sparrows). Female birds with brood patches were immediately released and excluded from the study to minimize impact on reproduction. Experimental feeding trials were conducted from 7:30 to 12:00 h (GMT + 1 h).
Individual birds were confined for 30 min in an insect cage (BugDorm-43030F, 32.5 × 32.5 × 32.5 cm, MegaView Science Co, Taichung, Taiwan) containing 53 ± 33.7 (mean ± SD) (range 1–152) female mosquitoes of either Cx. pipiens or Oc. caspius. Trials took place in a quiet, low-light environment to minimize behavioral alterations. Previous studies have shown that mosquito species, including Cx. pipiens, can feed on caged birds [39, 40]. Each bird was immobilized to prevent defensive behaviors against mosquitoes. Jackdaws were immobilized using non-permanent masking tape to attach wings to the body, close the beak, and hold legs together, leaving un-feathered areas (legs and eyes) exposed for mosquito feeding. House sparrows were immobilized in a 1 × 1 cm mesh cylinder, allowing mosquitoes to bite through. After trials, birds were released at their capture location without apparent harm. Mosquitoes with recent blood meals, including partial and full engorgement, were counted and classified as blood-fed.
Molecular Analyses
Genomic DNA was extracted from bird blood samples using the Maxwell® 16 LEV Blood DNA Kit (Promega, Madison, WI, USA) [41]. Birds were molecularly sexed following Griffiths et al. [42]. Avian Plasmodium infection status was assessed by amplifying a 478 bp fragment of the mitochondrial cytochrome b gene following Hellgren et al. [43]. Amplicon presence was verified in 1.8% agarose gels, and positive samples were sequenced using BigDye technology (Applied Biosystems, Foster City, CA, USA) or Macrogen sequencing service (Macrogen Inc., Amsterdam, Netherlands). Sequences were edited using Sequencher™ v.4.9 software (Gene Codes Corp., Ann Arbor, MI, USA) and assigned to parasite genus by comparison with the GenBank database (National Centre for Biotechnology Information). Only birds infected with avian Plasmodium were included; birds infected or co-infected with Haemoproteus or Leucocytozoon were excluded.
The occurrence of Cx. pipiens biotypes in the larvae collection area (La Cañada de los Pájaros) was characterized in another study. We analyzed 140 mosquitoes by amplifying the 5’ flanking region of the CQ11 microsatellite following [44, 45], finding Cx. p. pipiens, Cx. p. molestus, and hybrids (45%, 10.7%, and 44.3% frequency, respectively). Biotypes of mosquitoes in this study were not analyzed due to the large sample size.
Statistical Analysis
Chi-square tests compared the proportion of mosquitoes that bit house sparrows and jackdaws separately for each mosquito species. Generalized mixed linear models (GLMMs) with binomial error and logit link function were used to assess the effects of mosquito species and bird characteristics on mosquito biting rates, using R software v.3.2.5 [46] with the lme4 package [47]. First, we compared biting rates of the two mosquito species on birds. Models used mosquito biting rate as the dependent variable, expressed as the number of mosquitoes that bit versus those that did not, using the cbind function. Separate models were fitted for jackdaws and house sparrows due to immobilization method differences and body size variations. Each model included bird body mass and trial date (day 1 = Jan 1st) as covariates, and bird sex, Plasmodium infection status (infected/uninfected), and mosquito species (Cx. pipiens/Oc. caspius) as fixed factors. For house sparrows, captured in two years, year was included as a fixed factor. Two-way interactions between mosquito species and host sex and between mosquito species and infection status were also included. Bird identity was a random term to correct for overdispersion (dispersion parameter > 7.21) [48]. Body mass and date were scaled for each species by standard deviation and mean-centered for normalization. A medication experiment on jackdaws (primaquine or control) did not affect mosquito biting rate (Z = -1.2, Estimate = -0.62, P = 0.26), so this factor was excluded from further analyses. The potential effect of different Plasmodium lineages on biting rates was not analyzed due to small sample sizes for different bird/mosquito species combinations (Table 1).
Table 1.
Number of individuals infected with each Plasmodium lineage in this study
| Plasmodium lineages | Jackdaws | House sparrows |
|—|—|—|—|—|—|
| | Cx. pipiens | Oc. caspius | Cx. pipiens | Oc. caspius |
| SGS1 | 21 | 5 | 17 | 13 |
| GRW11 | 7 | 2 | | |
| COLL1 | 6 | 2 | | |
| PADOM01 | 3 | 2 | | |
| DELURB5 | 2 | 1 | | |
| PADOM02 | 3 | | | |
| GRW4 | 1 | | | |
| Total infected | 30 | | 55 | |
Results
The study included 72 jackdaws (34 males, 38 females) and 101 house sparrows (66 males, 35 females). Avian Plasmodium infection was present in 30 jackdaws (41.7%) and 55 house sparrows (54.5%). Seven Plasmodium lineages were identified (Table 1). A total of 9153 female mosquitoes were used: 6387 Cx. pipiens and 2766 Oc. caspius. Of these, 630 (9.9%) Cx. pipiens and 633 (22.9%) Oc. caspius blood-fed (Table 2), including 294 (46.7%) Cx. pipiens and 436 (68.9%) Oc. caspius on jackdaws, and 336 (53.3%) Cx. pipiens and 197 (31.1%) Oc. caspius on house sparrows (Table 2).
Table 2.
Summary data of mosquitoes biting jackdaws and house sparrows used in this study with respect to host sex and infection status by avian Plasmodium parasites
| n | No. of mosquitoes in each assay per box (mean ± SE) | No. of engorged mosquitoes per box (mean ± SE) |
|—|—|—|—|—|—|
| Jackdaws | | | | | |
| Cx. pipiens | Sex | Male | 26 | 59.1 ± 6.5 | 7.0 ± 2.6 |
| | Female | 29 | 49.7 ± 6.2 | 3.6 ± 1.0 |
| | Infectious status | Uninfected | 32 | 58.7 ± 5.9 | 4.9 ± 2.9 |
| | | Infected | 23 | 47.8 ± 7.1 | 6.0 ± 3.5 |
| Oc. caspius | Sex | Male | 8 | 62.9 ± 12.0 | 17.0 ± 4.8 |
| | Female | 9 | 58.1 ± 11.2 | 33.5 ± 6.2 |
| | Infectious status | Uninfected | 10 | 71.9 ± 10.7 | 21.3 ± 5.8 |
| | | Infected | 7 | 45.9 ± 12.8 | 30.3 ± 7.3 |
| House sparrows | | | | | |
| Cx. pipiens | Sex | Male | 41 | 57.3 ± 5.4 | 4.9 ± 0.9 |
| | Female | 20 | 53.0 ± 7.7 | 6.9 ± 1.3 |
| | Infectious status | Uninfected | 25 | 52.0 ± 6.8 | 4.6 ± 1.2 |
| | | Infected | 36 | 58.6 ± 5.8 | 6.2 ± 1.0 |
| Oc. caspius | Sex | Male | 25 | 44.4 ± 6.8 | 4.2 ± 1.2 |
| | Female | 15 | 41.7 ± 9.4 | 6.1 ± 1.6 |
| | Infectious status | Uninfected | 21 | 32.7 ± 7.5 | 3.6 ± 1.3 |
| | | Infected | 19 | 55.2 ± 8.2 | 6.4 ± 1.4 |
Oc. caspius bit jackdaws at a higher rate than house sparrows (χ2 = 15.43, df = 1, P < 0.001), while Cx. pipiens showed no significant difference (χ2 = 0.04, df = 1, P = 0.84; Fig. 1). The mammophilic Oc. caspius had a significantly higher biting rate than the ornithophilic Cx. pipiens (Z = 4.22, Estimate = 1.00, P < 0.001; Fig. 1).
Fig. 1.
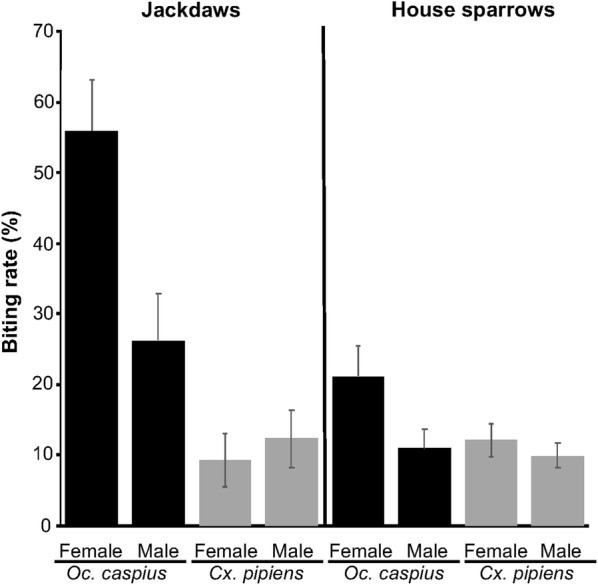 Biting rates of Ochlerotatus caspius and Culex pipiens mosquitoes on female and male jackdaws and house sparrows
Biting rates of Ochlerotatus caspius and Culex pipiens mosquitoes on female and male jackdaws and house sparrows
Biting rates of Ochlerotatus caspius and Culex pipiens mosquitoes on female and male jackdaws and house sparrows.
Bird trait effects on mosquito biting rates were analyzed separately for each bird species due to methodological differences (immobilization) and size variations. For jackdaws, Oc. caspius had significantly higher biting rates than Cx. pipiens (Table 3). Oc. caspius also bit female jackdaws more than males, while Cx. pipiens showed no significant sex-based difference (Table 3, Fig. 1). Host infection status, body mass, and date were not significantly related to mosquito biting rates on jackdaws (Table 3). For house sparrows, biting rates did not differ significantly between Cx. pipiens and Oc. caspius. Date was the only significant variable, with biting rates increasing as the season progressed. Host sex, body mass, and infection status were not significantly related to mosquito biting rates on house sparrows (Table 3, Fig. 1).
Table 3.
Results of GLMMs analyzing mosquito biting rates in relation to mosquito species (Ochlerotatus caspius and Culex pipiens) and birds’ body mass, sex, Plasmodium infection status, date on which each trial was conducted, and year of bird capture. Significant effects are highlighted in bold.
| Explanatory variables | Jackdaws | House sparrows |
|—|—|—|—|—|—|—|—|
| | Estimate | SE | Z-value | P-value | Estimate | SE | Z-value | P-value |
| Mosquito species | 2.51 | 0.56 | 4.50 | < 0.001 | 0.51 | 0.54 | 0.94 | 0.35 |
| Body mass | 0.75 | 0.82 | 0.92 | 0.36 | 1.46 | 0.80 | 1.84 | 0.07 |
| Sex | 0.04 | 0.39 | 0.10 | 0.92 | -0.57 | 0.36 | -1.59 | 0.11 |
| Infection status | 0.03 | 0.36 | 0.07 | 0.95 | 0.02 | 0.37 | 0.05 | 0.96 |
| Date trial | 0.86 | 0.82 | 1.04 | 0.30 | 2.11 | 0.71 | 2.96 | 0.003 |
| Year | – | – | – | – | 0.25 | 0.36 | 0.71 | 0.48 |
| Mosquito species*sex | -1.37 | 0.69 | -1.99 | 0.047 | 0.28 | 0.56 | 0.60 | 0.62 |
| Mosquito species*infection status | 0.83 | 0.70 | 1.19 | 0.23 | -0.44 | 0.57 | -0.77 | 0.44 |
| Explained variance (R2) | 0.20 | | | | 0.07 | | | |
Abbreviation: SE, standard error
Discussion
Understanding the causes of non-random mosquito biting patterns is crucial for comprehending avian Plasmodium and other vector-borne pathogen transmission dynamics [13]. This study examined how avian traits (body mass, sex, Plasmodium infection status) in two bird species affect biting rates of two mosquito species potentially involved in avian malaria transmission [32]. Future studies should also consider factors like blood meal size, which could influence parasite transmission.
The mammophilic Oc. caspius exhibited higher biting rates on jackdaws than the ornithophilic Cx. pipiens, while no significant difference was found for house sparrows. Although Oc. caspius primarily feeds on mammals, birds (including chickens and house sparrows) constitute 9.1–19.9% of their blood meals [6, 49], contrasting with Cx. pipiens, where birds account for 85.1–91.67% of blood meals [6]. Cx. pipiens biotypes were not considered, although feeding preference variations exist [50]. However, mosquitoes were collected in an area where both biotypes (Cx. pipiens pipiens and Cx. pipiens molestus) and hybrids coexist. A previous study in southern Spain found no significant differences in bird blood meal proportions between Culex pipiens biotypes [45]. Our results confirm Oc. caspius‘s ability to feed on birds, particularly when host choice is limited. This is relevant as our study focuses on biting rates, not feeding preferences. Unlike Cx. pipiens, Oc. caspius is considered aggressive and a human nuisance [51], though supporting experimental evidence is limited. Biting rate differences between species could be linked to life history traits and breeding needs, especially water source availability. Oc. caspius relies on tidal cycles and temporary flooded areas for larval development [52], whereas Cx. pipiens uses more permanent water sources [53], potentially making their life cycle less time-constrained. Differential activity patterns might also contribute, with Oc. caspius peaking during the day and Cx. pipiens at night/sunset [49]. While this could explain species differences, Cx. pipiens biting rates here are similar to those in nighttime experiments [30]. Seasonal climate variations affect host and vector phenology, potentially influencing host-vector interactions. We observed increased mosquito biting rates on house sparrows from spring to autumn. Edman [54] reported seasonal Culex nigripalpus feeding pattern changes. In our study, this effect seems host-mosquito assemblage-specific, seen in only one bird species. However, this variable’s relevance is low, explaining only 7% of variance.
Differences in biting patterns only appearing with jackdaws, the larger host, suggest a link to cue emission amounts. Visual and thermo-sensory stimulation becomes more important for mosquitoes closer to hosts. Multiple sensory cues can increase feeding success [3]. Larger jackdaws may emit more attractants (CO2, heat, odors) than house sparrows, causing the observed differences.
Prior studies show a positive correlation between host body mass and blood-sucking insect biting rates [19, 55], typically in interspecific comparisons. As expected, Oc. caspius bit larger jackdaws more than house sparrows, although Cx. pipiens showed no significant difference. Exposed skin area, usually linked to body mass, may affect mosquito feeding success [56]. Body mass is a key factor in West Nile virus prevalence in birds [57]. Yan et al. [58] found Cx. pipiens fed more on birds with longer tarsi, suggesting larger exposed skin areas are important. Burkett-Cadena et al. [39] found increased biting of nestlings as they grow, suggesting size and exposed skin influence feeding patterns. However, we found no significant intraspecific relationship between mosquito biting rates and bird body mass. Lalubin et al. [29] also found no association between Cx. pipiens attraction to house sparrows and their body mass. Intraspecific body mass differences may be less important at close range than cues like heat, humidity, or odor [3].
Host sex influenced Oc. caspius biting rates on jackdaws, but contrary to our prediction, females were preferred over males. No such difference was found for house sparrows, and Cx. pipiens biting rates were not sex-related. Burkett-Cadena et al. [25] found male-biased blood meals in mosquitoes (64% from males), possibly due to skewed wild bird sex ratios. However, significant sex-biased feeding patterns in bird-biting mosquitoes, including Culex species, are not generally observed [25]. Simpson et al. [20] concluded bird sex doesn’t affect Cx. pipiens choice between partners. Mammophilic mosquito preference for a specific bird sex could relate to sex-based odor profile differences. Preen gland secretions, containing volatile and non-volatile substances, can influence blood-sucking insect feeding preferences [59, 60], and their composition varies between bird sexes [61, 62]. Differential responses of Oc. caspius and Cx. pipiens to preen gland secretions could partly explain mosquito species discrepancies [63].
We found no evidence linking avian Plasmodium infection status to mosquito biting rates. It remains unclear whether avian malaria infection increases [26, 27] or decreases [29] mosquito attraction to infected hosts. The host manipulation hypothesis, suggesting Plasmodium enhances transmission by increasing host attractiveness, remains unresolved [64, 65]. Table 4 summarizes studies on avian infections and mosquito attraction, showing varied methodologies (olfactometers, immobilized birds) and host-vector assemblages, potentially affecting conclusions. Our study used naturally infected birds with diverse Plasmodium lineages and infection stages, which could influence host attractiveness. Low prevalence of some lineages prevented analysis of lineage-specific effects on mosquito feeding preference. However, no prior study has found differential bird attractiveness based on infecting Plasmodium lineage. Besides cue emission changes in infected hosts [70], infection-related changes in defensive behavior could affect mosquito susceptibility [68, 69]. Day et al. [68] found malaria-infected mice were more lethargic and less defensive. In our study, birds showed no lethargy and were immobilized, eliminating defensive behavior effects. Potential cue emission differences between infected and uninfected hosts [70] may only be detectable at longer distances, where host-seeking relies mainly on olfactory cues [3], or in dual-choice experiments [26], unlike our study design.
Table 4.
Studies on the effects of avian malaria and malaria-like infections in birds on the biting patterns of different insect vectors.
| Effects | Vectors | Bird species | Parasite species | Methods | Results | Reference |
|---|---|---|---|---|---|---|
| Positive | Cx. pipiens | Canaries | P. relictum SGS1 | Biting rate on immobilized birds exposed in pairs | Infected birds more bitten | [26] |
| Positive | Cx. pipiens | Canaries | P. relictum SGS1 | Biting rate on immobilized birds exposed in pairs | Infected birds more bitten | [27] |
| Positive | Cx. pipiens | House sparrows | Plasmodium | Biting rate on birds exposed in pairs | Infection intensity, not prevalence, determined biting rates | [30] |
| Negative | Cx. pipiens | Great tits | Plasmodium | Attractivity of birds exposed in pairs (olfactometer) | Uninfected birds more attractive | [29] |
| Negative | Biting midges; blackflies | Blue tits | Haemoproteus majoris | Insect abundance in nest boxes | Higher Culicoides abundance in nests of medicated females | [66] |
| Negative | Biting midges | Blue tits | Haemoproteus | Insect abundance in nest boxes | Higher C. festivipennis abundance in nests from medicated birds | [67] |
| No effect | Cx. pipiens; Oc. caspius | House sparrows; jackdaws | Plasmodium | Biting rate on immobilized birds exposed individually | No infection status effect on biting rate | This study |
Conclusions
This study emphasizes that host trait effects on mosquito feeding patterns are not easily generalized. Only host sex showed an association with biting rate differences, and only for one mosquito species. Mosquito biting patterns vary based on vector and host species characteristics. Mammophilic mosquito preference for a specific sex remains unclear and requires further investigation, particularly regarding olfactory cues from male and female birds.
Acknowledgements
We thank A. Díez, M. Ferraguti, L. Gómez, I. Martín, A. Pastoriza, E. Pérez, S. Ruiz and J. Yan for their assistance. P. Rodríguez and M. Adrián facilitated mosquito larvae and bird sampling in “La Cañada de los Pájaros”. We appreciate the constructive feedback from two anonymous reviewers.
Funding
This work was supported by projects CGL2012-30759 and CGL2015-65055-P from the Spanish Ministerio de Economía y Competitividad and European Regional Development’s funds (FEDER), and project P11-RNM-7038 from the Junta de Andalucía. RGL was funded by a FPI grant (BES-2013-065274). JMP was partially supported by a Juan de la Cierva contract and a 2017 Leonardo Grant for Researchers and Cultural Creators, BBVA Foundation. LG was supported by a Marie Curie Fellowship (grant number 747729 “EcoEvoClim”).
Availability of data and materials
Data are available within the article and from the corresponding author upon request.
Authors’ contributions
All authors designed the study. RGL and JMP conducted experiments and lab analyses. RGL, JMP, LG, and JF performed statistical analyses. All authors read and approved the manuscript.
Ethics approval and consent to participate
All procedures were approved by the CSIC Ethics committee and Animal Health authorities (439-2016) and complied with Spanish laws.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral on jurisdictional claims.
Contributor Information
Rafael Gutiérrez-López, Email: [email protected].
Josué Martínez-de la Puente, Email: [email protected].
Laura Gangoso, Email: [email protected].
Ramón Soriguer, Email: [email protected].
Jordi Figuerola, Email: [email protected].
References
[1] Takken W, Knols BGJ. Odor-mediated host-seeking behavior of mosquitoes. Annu Rev Entomol. 1999;44(1):131–57.
[2] Gillies MT. Visual responses of flying mosquitoes to the human face. Bull Entomol Res. 1980;70(2):257–68.
[3] Dekker T, ter Braak CJF. Host preference of mosquitoes and the multi-sensory integration of host cues. Entomol Exp Appl. 2011;141(2):117–29.
[4] Tempelis CH. Host preferences of mosquitoes. Annu Rev Entomol. 1975;20(1):183–201.
[5] Kay BH, Boreham PFL, Edman JD. Blood-feeding patterns of mosquitoes in southeastern Queensland, Australia. J Med Entomol. 1979;16(2):115–23.
[6] Sánchez-Vargas I,规律