CRISPR, or Clustered Regularly Interspaced Short Palindromic Repeats, is a revolutionary genome editing technology derived from the adaptive immune response of bacteria. At CONDUCT.EDU.VN, we offer expert guidance to help you understand the core components and applications of CRISPR, ensuring you can effectively leverage this powerful tool. Discover how guide RNA functions within CRISPR systems to target specific genes and realize the full potential of CRISPR technology with our comprehensive resources on gene editing, ethical guidelines, and regulatory compliance.
1. Introduction to Guide RNA in CRISPR
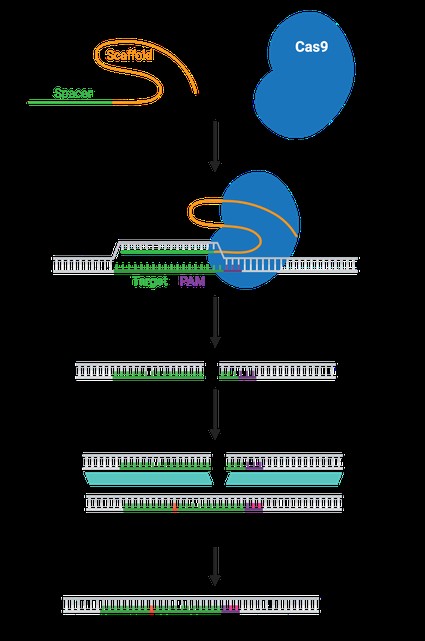
CRISPR-Cas9 technology has transformed the field of genetic engineering due to its precision and versatility. The guide RNA (gRNA) is a critical component, acting as the GPS that directs the Cas9 enzyme to the precise location in the genome where editing is desired. Understanding the role and function of gRNA is essential for anyone working with CRISPR.
1.1. Defining Guide RNA (gRNA)
A guide RNA (gRNA) is a short, synthetic RNA molecule composed of two key regions:
- scaffold sequence: Necessary for Cas9-binding.
- spacer sequence: A user-defined ~20-nucleotide sequence that determines the genomic target.
The gRNA essentially programs the Cas9 enzyme to target a specific DNA sequence, enabling precise genome editing. This programmability makes CRISPR highly adaptable, as changing the spacer sequence allows researchers to target different genes with relative ease.
1.2. The Essential Role of gRNA in CRISPR-Cas9
The gRNA plays a pivotal role in the CRISPR-Cas9 system by:
- Targeting: Guiding the Cas9 enzyme to the specific DNA sequence intended for modification.
- Specificity: Ensuring that Cas9 only binds to the intended site, reducing off-target effects.
- Adaptability: Allowing researchers to easily change the target site by modifying the spacer sequence.
Without the gRNA, Cas9 would not be able to find and bind to the correct location in the genome, rendering the CRISPR system ineffective.
1.3. Understanding gRNA Structure: Scaffold and Spacer
To fully appreciate how gRNA functions, it’s essential to understand its structural components:
- Scaffold Sequence: This region is crucial for binding to the Cas9 enzyme. It provides the necessary structure for the Cas9 protein to recognize and form a stable complex with the gRNA.
- Spacer Sequence: Also known as the targeting sequence, this is a 20-nucleotide sequence complementary to the DNA target site. The specificity of the spacer sequence determines where Cas9 will bind and cut the DNA.
1.4. The Significance of PAM in CRISPR Targeting
The Protospacer Adjacent Motif (PAM) is a short DNA sequence that must be present immediately adjacent to the target sequence for Cas9 to bind and cleave DNA. The most common PAM sequence for SpCas9 (from Streptococcus pyogenes) is NGG, where N can be any nucleotide. The PAM sequence acts as a binding signal for Cas9, ensuring that the enzyme only binds to the correct sites. Different Cas enzymes recognize different PAM sequences, providing flexibility in target selection.
2. Designing an Effective Guide RNA
Designing an effective guide RNA is crucial for successful CRISPR experiments. Several factors must be considered to ensure high on-target activity and minimal off-target effects.
2.1. Key Considerations for gRNA Design
When designing a gRNA, consider the following:
- Specificity: Ensure the 20-nucleotide spacer sequence is unique to the target gene and has minimal homology to other regions in the genome.
- PAM Proximity: The target sequence must be immediately adjacent to the appropriate PAM sequence for the Cas enzyme being used.
- GC Content: Aim for a GC content of 40-60% in the spacer sequence to ensure stable binding to the target DNA.
- Avoid PolyT Stretches: Avoid long stretches of thymines (T) in the spacer sequence, as these can act as premature termination signals for RNA polymerase.
2.2. Tools and Resources for gRNA Design
Several online tools and databases can aid in gRNA design:
- CRISPR Design Tool (MIT): Provides gRNA sequences for various genomes with off-target analysis.
- Benchling: A comprehensive platform for designing and managing CRISPR experiments.
- IDT CRISPR Design Tool: Offers optimized gRNA designs and synthesis services.
These tools help researchers identify gRNAs with high specificity and minimal off-target potential, improving the efficiency and accuracy of CRISPR editing.
2.3. Maximizing On-Target Activity and Minimizing Off-Target Effects
To maximize on-target activity and minimize off-target effects:
- Choose High-Specificity gRNAs: Select gRNAs with minimal homology to other regions in the genome.
- Use Engineered Cas Enzymes: High-fidelity Cas9 variants (hfCas9) have been engineered to reduce off-target cutting.
- Optimize gRNA Concentration: Titrate the amount of gRNA and Cas9 to find the optimal concentration for editing.
- Employ Paired Nickases: Use Cas9 nickases with two gRNAs targeting opposite strands of DNA to create a double-strand break (DSB) only at the intended site.
2.4. Validating gRNA Activity
After designing and synthesizing a gRNA, it’s crucial to validate its activity:
- Cleavage Assay: Perform an in vitro cleavage assay to confirm that the gRNA-Cas9 complex can cut the target DNA.
- Cell-Based Assay: Transfect cells with the gRNA and Cas9, then analyze the genomic DNA for editing efficiency using methods like T7E1 assay or next-generation sequencing (NGS).
- Phenotypic Analysis: Assess whether the intended gene knockout or edit results in the expected phenotypic changes.
Validating gRNA activity ensures that the CRISPR system is working as expected and helps identify any potential issues with the gRNA design or delivery.
3. Methods for gRNA Delivery
Efficient gRNA delivery is essential for successful CRISPR editing. Various methods can be used to deliver gRNA into cells, each with its own advantages and disadvantages.
3.1. Plasmid Delivery
Plasmid delivery involves cloning the gRNA sequence into a plasmid vector under the control of a promoter, such as U6 or H1. The plasmid is then introduced into cells using transfection methods like electroporation, lipofection, or viral transduction.
- Advantages: Easy to design and construct, can deliver both gRNA and Cas9.
- Disadvantages: Potential for prolonged expression, which may increase off-target effects.
3.2. RNA Delivery
RNA delivery involves synthesizing the gRNA in vitro and introducing it into cells as RNA molecules. This can be done using transfection methods or electroporation.
- Advantages: Transient expression, reduced off-target effects, suitable for applications requiring precise timing.
- Disadvantages: Can be more challenging to synthesize and deliver, requires co-delivery of Cas9 protein.
3.3. Viral Delivery
Viral delivery involves packaging the gRNA sequence into a viral vector, such as adeno-associated virus (AAV) or lentivirus. The virus is then used to transduce cells, delivering the gRNA.
- Advantages: High efficiency, can target a wide range of cell types, suitable for in vivo applications.
- Disadvantages: Potential for immunogenicity, insertional mutagenesis, and limited cargo capacity.
3.4. Ribonucleoprotein (RNP) Delivery
RNP delivery involves forming a complex of the gRNA and Cas9 protein in vitro and introducing the complex directly into cells. This can be done using transfection methods or electroporation.
- Advantages: High efficiency, transient activity, minimal off-target effects, suitable for precise genome editing.
- Disadvantages: Requires purified Cas9 protein, can be more expensive.
3.5. Choosing the Right Delivery Method
The choice of delivery method depends on the specific application and experimental goals:
- For stable gene knockouts or edits, plasmid or viral delivery may be suitable.
- For transient gene editing or applications requiring precise timing, RNA or RNP delivery may be preferred.
- For in vivo applications, viral delivery is often the most effective method.
4. Applications of CRISPR Using gRNA
CRISPR technology, guided by gRNA, has a wide range of applications in basic research, biotechnology, and medicine.
4.1. Gene Knockout
Gene knockout involves disrupting a gene’s function, typically by introducing insertions or deletions (indels) at the target site. This can be achieved by delivering a gRNA and Cas9 to a cell, leading to a double-strand break (DSB) that is repaired by non-homologous end joining (NHEJ), an error-prone repair pathway.
- Applications: Studying gene function, creating disease models, developing new therapies.
4.2. Gene Knock-in
Gene knock-in involves inserting a specific DNA sequence into a target site. This requires delivering a gRNA, Cas9, and a donor template containing the desired sequence flanked by homology arms. The DSB is repaired by homology-directed repair (HDR), using the donor template as a guide.
- Applications: Introducing specific mutations, adding tags or reporters, creating genetically modified organisms.
4.3. Gene Editing
Gene editing involves making precise changes to a DNA sequence. This can be achieved using base editing or prime editing, which do not require DSBs.
-
Base Editing: Converts a single base pair to another (e.g., C to T or A to G) using a Cas9 nickase fused to a deaminase enzyme.
-
Prime Editing: Inserts or deletes short sequences or makes all possible base-to-base conversions using a Cas9 nickase fused to a reverse transcriptase.
-
Applications: Correcting genetic mutations, creating new genetic variants, studying gene function.
4.4. Gene Regulation
CRISPR can be used to regulate gene expression without permanently altering the DNA sequence. This can be achieved using catalytically inactive Cas9 (dCas9) fused to transcriptional activators or repressors.
-
CRISPR Activation (CRISPRa): Activates gene expression by recruiting transcriptional activators to the target site.
-
CRISPR Interference (CRISPRi): Represses gene expression by blocking transcription initiation or elongation.
-
Applications: Studying gene regulation, developing new therapies, creating synthetic biological circuits.
4.5. Genome-Wide Screening
CRISPR can be used to screen for genes involved in specific cellular processes or phenotypes. This typically involves using a library of gRNAs targeting different genes, introducing the library into cells, and selecting for cells with the desired phenotype.
- Applications: Identifying drug targets, studying disease mechanisms, understanding cellular signaling pathways.
4.6. Diagnostics
CRISPR-based diagnostics can be used to detect specific DNA or RNA sequences in a sample. This typically involves using a Cas enzyme that cleaves a reporter molecule upon binding to the target sequence, generating a detectable signal.
- Applications: Detecting pathogens, diagnosing genetic diseases, monitoring gene expression.
5. Enhancing CRISPR Specificity and Efficiency
Improving the specificity and efficiency of CRISPR editing is crucial for minimizing off-target effects and maximizing on-target activity.
5.1. High-Fidelity Cas Enzymes
Engineered Cas9 variants, such as hfCas9 and eSpCas9, have been developed to reduce off-target cutting. These variants have mutations that disrupt interactions between Cas9 and DNA, increasing the enzyme’s proofreading capabilities.
5.2. Paired Nickases
Using Cas9 nickases with two gRNAs targeting opposite strands of DNA can reduce off-target effects. The DSB is only created at the intended site, minimizing the chance of off-target cutting.
5.3. Modified gRNA Designs
Modifying the gRNA sequence or structure can improve specificity. This includes truncating the gRNA to 17-18 nucleotides or adding chemical modifications to the gRNA.
5.4. Chemical Modifications
Adding chemical modifications to the gRNA, such as 2′-O-methyl modifications or phosphorothioate linkages, can improve its stability and reduce off-target effects.
5.5. CRISPR Inhibitors
Anti-CRISPR (Acr) proteins can be used to control CRISPR activity and reduce off-target effects. Acr proteins inhibit CRISPR by blocking crRNA loading, PAM recognition, or Cas9’s endonuclease domain.
5.6. Optimizing Delivery Methods
Choosing the appropriate delivery method can improve efficiency. RNP delivery, for example, offers high efficiency and transient activity, minimizing off-target effects.
6. Ethical Considerations in CRISPR Technology
The use of CRISPR technology raises several ethical considerations that must be carefully addressed.
6.1. Germline Editing
Germline editing involves making changes to the DNA of germ cells (sperm or eggs), which can be passed on to future generations. This raises concerns about unintended consequences and the potential for creating inheritable genetic modifications.
6.2. Somatic vs. Germline Editing
- Somatic Editing: Changes are made to non-reproductive cells and are not passed on to future generations.
- Germline Editing: Changes are made to reproductive cells and are passed on to future generations.
6.3. Informed Consent
Ensuring that patients and research participants fully understand the risks and benefits of CRISPR-based therapies is crucial. Informed consent should be obtained before any CRISPR-based interventions are performed.
6.4. Equity and Access
Ensuring that CRISPR-based therapies are accessible to all individuals, regardless of socioeconomic status, is essential. Addressing issues of equity and access is crucial for preventing disparities in healthcare.
6.5. Regulation and Oversight
Establishing clear regulations and oversight mechanisms is crucial for ensuring the responsible use of CRISPR technology. This includes setting standards for research, development, and clinical applications.
CONDUCT.EDU.VN is committed to promoting ethical practices and providing resources for understanding and addressing the ethical considerations in CRISPR technology. Our goal is to foster responsible innovation and ensure that CRISPR is used for the benefit of all.
7. Future Directions and Innovations in gRNA Technology
The field of gRNA technology is rapidly evolving, with ongoing research focused on improving specificity, efficiency, and delivery methods.
7.1. Next-Generation gRNA Designs
Researchers are developing new gRNA designs that incorporate chemical modifications, truncated sequences, or optimized structures to improve specificity and reduce off-target effects.
7.2. Enhanced Delivery Methods
New delivery methods, such as lipid nanoparticles (LNPs) and exosomes, are being developed to improve the efficiency and safety of gRNA delivery.
7.3. Multiplex CRISPR
Multiplex CRISPR involves using multiple gRNAs to target multiple genes simultaneously. This allows researchers to study complex biological processes and develop new therapies for multi-genic diseases.
7.4. CRISPR-Based Diagnostics
CRISPR-based diagnostics are being developed for a wide range of applications, including detecting pathogens, diagnosing genetic diseases, and monitoring gene expression.
7.5. Therapeutic Applications
CRISPR-based therapies are being developed for a wide range of diseases, including cancer, genetic disorders, and infectious diseases.
8. FAQ: Guide RNA in CRISPR
Here are some frequently asked questions about guide RNA in CRISPR:
-
What is the role of guide RNA (gRNA) in CRISPR-Cas9?
- gRNA guides the Cas9 enzyme to the specific DNA sequence intended for modification.
-
How is gRNA designed?
- gRNA consists of a scaffold sequence for Cas9 binding and a 20-nucleotide spacer sequence complementary to the target DNA.
-
What is PAM?
- Protospacer Adjacent Motif (PAM) is a short DNA sequence required for Cas9 binding and cleavage.
-
How can off-target effects be minimized?
- Use high-fidelity Cas9 variants, paired nickases, modified gRNA designs, and CRISPR inhibitors.
-
What are the different methods for gRNA delivery?
- Plasmid delivery, RNA delivery, viral delivery, and ribonucleoprotein (RNP) delivery.
-
What are the applications of CRISPR using gRNA?
- Gene knockout, gene knock-in, gene editing, gene regulation, genome-wide screening, and diagnostics.
-
What are the ethical considerations in CRISPR technology?
- Germline editing, informed consent, equity and access, and regulation and oversight.
-
How does base editing work?
- Base editing uses a Cas9 nickase fused to a deaminase enzyme to convert a single base pair to another.
-
What is prime editing?
- Prime editing uses a Cas9 nickase fused to a reverse transcriptase to insert or delete short sequences or make base-to-base conversions.
-
What is CRISPRi and CRISPRa?
- CRISPRi (CRISPR interference) represses gene expression, while CRISPRa (CRISPR activation) activates gene expression.
9. Conclusion: Mastering gRNA for CRISPR Success
Understanding the role of guide RNA in CRISPR technology is crucial for successful genome editing. By carefully designing gRNAs, optimizing delivery methods, and addressing ethical considerations, researchers can leverage the full potential of CRISPR for basic research, biotechnology, and medicine.
CONDUCT.EDU.VN provides comprehensive resources and guidance to help you navigate the complexities of CRISPR technology. Whether you are a student, researcher, or industry professional, our goal is to empower you with the knowledge and tools you need to succeed in the field of genome editing.
Ready to dive deeper? Explore more articles and resources on CONDUCT.EDU.VN to enhance your understanding of CRISPR technology and its applications. Contact us at 100 Ethics Plaza, Guideline City, CA 90210, United States, or via Whatsapp at +1 (707) 555-1234 for personalized guidance and support. Visit our website at conduct.edu.vn today.