The development of a functional nervous system hinges on the precise navigation of axons to their appropriate postsynaptic partners. This journey, which can span from micrometers to meters, occurs within a complex extracellular environment characterized by diverse mechanical and chemical cues. The intricate networks of nerve fibers and axons observed in mature organisms are a testament to the sophisticated pathfinding mechanisms that guide their formation. Remarkably, the macroscopic structure of axon projections is highly conserved across individuals of the same species, suggesting the existence of precise guidance mechanisms. As axons develop, they exhibit directionally biased growth, either towards or away from external guidance cues. Netrin-1 is a prominent guidance cue, whose presentation in vivo continues to be a subject of discussion. These guidance cues can manifest as secreted soluble or chemotactic gradients, or they can be bound to cells or the extracellular matrix, creating haptotactic gradients. The growth cone, a specialized and highly dynamic structure at the tip of the extending axon, detects these cues via transmembrane receptors such as deleted in colorectal cancer (DCC) and UNC5. These receptors orchestrate the remodeling of the cytoskeleton and cell membrane through chemical and mechanotransductive pathways, resulting in traction forces generated by the cytoskeleton against the extracellular environment and subsequent growth cone translocation. Netrin-1, through intracellular signaling responses, can either attract or repel the axon. This review delves into the mechanisms by which netrin-1, a classical guidance cue, regulates intracellular effectors to respond to the extracellular environment in the context of axon guidance during development of the central nervous system. We also explore recent discoveries highlighting the critical role of mechanical forces in this process.
Axon’s Response to the Extracellular Milieu
The development of an animal’s nervous system, irrespective of its complexity, requires that each neuron establish connections with the correct target cells. This feat is achieved through the extension of a specialized projection, known as the axon, from the neuronal cell body. The growing axon embarks on a journey that can cover relatively long distances, sometimes exceeding several thousand times the cell body’s diameter. Ultimately, the axon reaches the correct location, giving rise to the stereotyped circuits observed within a species.
The growth cone, a complex, cytoskeletal-rich structure located at the end of the developing axon, assumes responsibility for extending the axon and detecting and responding to the extracellular signals that guide pathfinding. These signals, often in the form of glycoproteins secreted into or presented attached to the extracellular matrix, act as ligands for receptors on the surface of the growth cone. These ligands trigger a cascade of intracellular responses, including membrane remodeling through exocytosis and endocytosis, cytoskeletal reorganization, and modification of protein expression and degradation, both locally within the axon and throughout the neuron.
For in-depth and up-to-date insights into growth cone regulation, explore the following: “Regulation of plasma membrane expansion during axon formation” on membrane remodeling and addition (Quiroga et al., 2017), “Actin based growth cone motility and guidance” on actin responses in the growth cone (Omotade et al., 2017), “Mechanochemical regulation of growth cone motility” on mechanosensation and mechanotransduction by growth cones (Kerstein et al., 2015), and “Axon Guidance Pathways and the Control of Gene Expression” on the regulation of gene expression (Russell and Bashaw, 2018). This review will focus on the mechanisms by which Who Guides, specifically netrin-1, leads to axon guidance responses.
Netrin-1: The Quintessential Guidance Cue
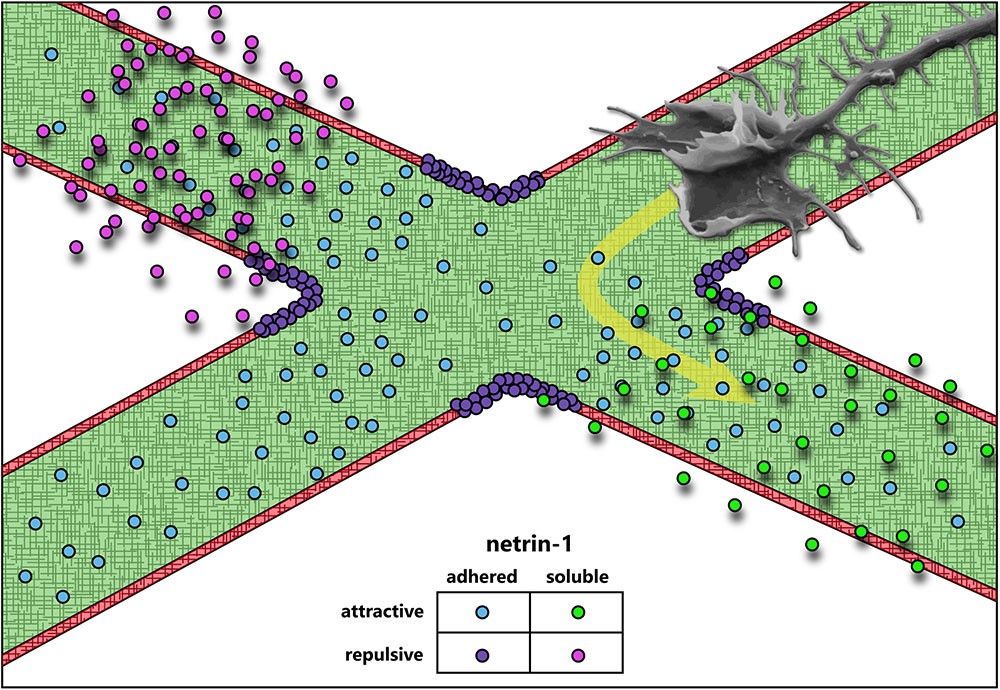
One of the groundbreaking discoveries in the field of axon guidance revealed that axonal outgrowth, promoted by a previously unknown and presumably diffusible extracellular cue, exhibited a bias in the direction of the cue source (Serafini et al., 1994). Purification of the major factors from chick brain that promoted axon outgrowth in embryonic rat spinal cord explants yielded two proteins homologous to the C. elegans unc-6 gene product required for axon guidance (Hedgecock et al., 1990). These proteins were named netrin-1 and netrin-2, drawing inspiration from the Sanskrit word “netr,” meaning “one who guides” (Serafini et al., 1994). Subsequent research confirmed their role as axonal guidance cues (Kennedy et al., 1994; Deiner et al., 1997; Mehlen and Rama, 2007; Moore et al., 2007; Masuda et al., 2009; Xu et al., 2010; Sun et al., 2011). Since then, netrin-1 has become one of the most extensively studied proteins in its class, with known functions in axon guidance, axon branching (Dent et al., 2004), synaptogenesis (Flores, 2011), cell migration (Ylivinkka et al., 2016), cell survival (Mehlen and Furne, 2005), and axon regeneration (Dun and Parkinson, 2017). This review will primarily focus on the function of netrin-1 in the context of axon guidance. Netrin-1-mediated axon guidance has been implicated in the development of multiple brain regions and neuronal types, solidifying its status as one of the most characterized and diversely functioning guidance cues. Interestingly, while many axon guidance cues predominantly function as either attractive or repulsive, and as either diffusible/chemotactic or adhesive/haptotactic molecules, netrin-1’s function has never neatly fit into a single category (Figure 1). This diversity makes netrin-1 an ideal candidate for mechanotransduction studies in axon guidance, particularly as recent studies have emphasized the importance of substrate adhesion in netrin-1 function in vivo (Dominici et al., 2017; Varadarajan et al., 2017; Yamauchi et al., 2017).
Attraction and Repulsion
Even in the earliest descriptions of the C. elegans genes unc-5 (UNC5 in mammals), unc-6 (NTN1 in mammals), and unc-40 (DCC and NEO in mammals, frazzled in Drosophila), evidence suggested that a ventral source of unc-6 both attracts and repulses axons (Hedgecock et al., 1990). Deletion of unc-6 affects guidance of axons that extend dorsally (repulsion) or ventrally (attraction). Dorsal guidance is specifically impaired by deletion of unc-5 and ventral guidance is impaired by deletion of unc-40, suggesting the different responses are orchestrated by distinct receptors. Later experiments using chick spinal explants describe a bimodal axon outgrowth response to increasing concentrations of netrin-1 (Serafini et al., 1994), with the highest concentrations promoting less robust outgrowth. A concentration-dependent bimodal response is also observed in the turning of embryonic cortical murine axons in a stable gradient of netrin-1 in vitro (Taylor et al., 2015). Elegant experiments by a number of labs over the years have established that this bifunctionality of netrin-1 signaling is dependent upon the receptors presented by the axonal growth cone. Netrin binding to the receptor deleted in colorectal cancer (DCC) results in attractive responses, via homodimerization of DCC (covered in detail in later sections) (Chan et al., 1996; Keino-Masu et al., 1996; Kolodziej et al., 1996; Fazeli et al., 1997), whereas heterodimerization between DCC and receptor uncoordinated locomotion 5 (UNC5) converts this attractive response into repulsion (Hamelin et al., 1993; Colavita and Culotti, 1998; Finci et al., 2014). Intriguingly, UNC5 can also mediate shorter-range repulsive responses to netrin-1 in the absence of DCC (Keleman and Dickson, 2001). This repulsive response requires association between UNC5 and the co-receptor, down syndrome cell adhesion molecule (DSCAM) (Purohit et al., 2012). The structures and outcomes of known netrin-1 receptor dimers are summarized in Figure 2.
An important feature of many netrin-1 signaling pathways, both attractive and repulsive, appears to be interaction between the cytoplasmic domains of dimerized receptors (Figure 2). Netrin-1 induces homodimerization of DCC, bringing their cytoplasmic tails into close proximity (Finci et al., 2014). The close apposition of these domains is thought to create an assembly platform for the association of further signaling effectors. In the case of netrin-induced repulsion, the association of the intracellular P1 domain of DCC and a DCC-binding domain of UNC5 is also required (Figure 2) (Hong et al., 1999). Whether netrin-1 repulsion by an UNC5/DSCAM complex analogously involves association between intracellular domains of these two receptors remains to be determined. Though the repertoire of receptors that govern the attractive or repulsive responses to netrin-1 have been identified, the differences in intracellular signaling and mechanotransduction responses between these two modes are comparatively less well understood.
Attraction: Repulsion Switching
These experiments leave us with a glaring question: what determines whether exposure to netrin-1 results in attraction or repulsion of an axon? There are several possibilities for this “switch”; (1) the relative levels of each netrin-1 receptor on the surface of the growth cone; (2) a secondary signal of intracellular status (such as Ca2+, cGMP, or cAMP) that may activate or inhibit signaling pathway components, favoring either attraction or repulsion; (3) the relative affinities of individual receptor types for netrin-1, along with the extracellular concentration of netrin-1; or (4) the presence of other molecules in the extracellular environment. Current evidence suggests that all four of these mechanisms are capable of switching netrin-1 responses between attraction and repulsion. Known mechanisms that convert such netrin-1 responses are summarized in Figure 3.
Switch 1: Membrane Receptor Levels
Modulation of receptor expression levels or presentation on the surface of cells is a common mechanism for tuning responses to extracellular ligands (Groc and Choquet, 2006; Winckler and Yap, 2011; Ceresa, 2012; Hausott and Klimaschewski, 2016; Suh et al., 2018). Altered expression levels and/or surface levels of netrin receptors have also been implicated in modulating response to netrin-1. For example, the DNA repair gene Rad51 upregulates expression of UNC5B and UNC5C in mouse primary cortical neurons, and negatively regulates netrin-dependent axon branching (Glendining et al., 2017), typically viewed as an attractive response to netrin. Opposing this, endocytic internalization and membrane depletion of UNC5, and not DCC, converts repulsive netrin-1 responses to attraction. This UNC5 internalization is triggered by protein interacting with C-kinase 1 (PICK1)-dependent recruitment of active protein kinase Cα (PKCα) to the plasma membrane, which phosphorylates UNC5 residues S408 and S587 (Williams et al., 2003; Bartoe et al., 2006). Multiple studies suggest that surface levels of DCC are altered by exposure to netrin-1, however, the specific response varies between studies. In embryonic rat spinal commissural and cortical neurons, exposure to netrin-1 increases DCC localization to the plasma membrane (Matsumoto and Nagashima, 2010) potentiated by, but not requiring, protein kinase A (PKA) activation (Bouchard et al., 2004, 2008). A single study in dissociated rat embryonic cortical neurons suggests that DCC is ubiquitinated, internalized, and then degraded after netrin-1 exposure (Kim et al., 2005). However, whether this affects subsequent netrin-1 responses was not shown. Intriguingly KCl-induced depolarization of cortical neurons, but not spinal commissural neurons, leads to increased plasma membrane levels of DCC and increases axon outgrowth in response to netrin-1. These responses require activity of PKA, PKC, and phosphatidylinositol-3-kinases (PI3Ks), as well as exocytosis, but not protein synthesis (Bouchard et al., 2008). Additional studies are required to define how expression levels, surface localization, and protein stability of DCC are modulated by netrin in diverse cell types, and in scenarios in which netrin is perceived by the growth cone as attractive or repulsive.
Switch 2: Intracellular Secondary Messengers
Intracellular levels of the secondary messenger, cAMP, which promotes the activity of PKA, may trigger an attractive-repulsive switch in response to netrin. Inhibition of PKA in Xenopus spinal neurons with small molecules KT5720 or Rp-cAMPS causes a typically attractive netrin-1 gradient to repulse axons (Ming et al., 1997). The authors conducted a dose-response experiment using Rp-cAMPS, a potent and specific competitive inhibitor of cAMP-dependent activation of PKA, to investigate whether the change from netrin-1 attraction to repulsion was “switch” or a “dial.” This revealed that the turning response transitions abruptly between 1 and 5 μM Rp-cAMPS, rapidly plateauing at higher concentrations: such a sigmoidal response suggests a switch-like mechanism (Ming et al., 1997). Later experiments found that PKA inhibition with a considerably higher dose of KT5720 reduces attractive responses to netrin-1 in rat spinal commissural neurons in a spinal explant, but does not switch the response to repulsion (Moore and Kennedy, 2006). The differences in experimental conditions make interpretation of these data difficult, however, they imply that either higher concentration of PKA inhibitor, some non-neuronal component of the spinal explant, or differences between rat and frog spinal neurons, such as potential differing expression of netrin-1 receptor isoforms, alters the role of PKA in the netrin-1 attraction/repulsion switch.
Netrin-1 response is modulated by other secondary messengers in addition to cAMP/PKA. Axon guidance experiments in isolated Xenopus spinal commissural neurons revealed that attractive netrin-1 response relied on both Ca2+ release from the endoplasmic reticulum and Ca2+ influx through the plasma membrane, and that blockade of Ca2+ influx into the cytoplasm converted the attractive response to repulsion (Hong et al., 2000). Ca2+-dependent repulsion also requires cGMP. Using cyclic nucleotide analogs, Nishiyama et al. (2003) found that the ratio of [cAMP]/[cGMP] tunes netrin-dependent turning responses; with a high ratio promoting attraction and a low ratio leading to repulsion. Revisiting these classic experiments on netrin-1 attraction and repulsion with new tools to optogenetically manipulate the spatial and temporal distribution of secondary messenger levels is warranted.
Switch 3: Netrin-1 Concentration
Many axon guidance or outgrowth studies reveal bimodal responses of axons occur with increasing netrin concentrations (Serafini et al., 1994; Deiner et al., 1997; De La Torre et al., 1997; Ming et al., 1997; Moore and Kennedy, 2006), suggesting that the concentration of netrin-1 may determine which receptors, and thus intracellular pathways, are recruited. Indeed in a microfluidically isolated gradient of netrin-1, embryonic murine cortical axons closer to the source of netrin-1 (higher concentration) are repelled, whereas those at the lower end of the concentration gradient are attracted (Taylor et al., 2015), supporting the notion of a concentration dependent response. Although further work is needed to establish this mechanism, biophysical experiments have demonstrated that netrin-1 binds with higher affinity to DCC than to UNC5 (Finci et al., 2014). This could lead to increased UNC5/DCC heterodimerization at higher concentrations of netrin-1, inducing repulsive axon guidance responses (Hamelin et al., 1993; Colavita and Culotti, 1998; Finci et al., 2014). The attractive and repulsive forces leading to directional axon outgrowth under this paradigm are summarized in Figure 4. Structural studies on the interaction between netrin-1, DCC and UNC5 suggest that although DCC can bind two sites on netrin-1, only one of these can interact with UNC5 (Finci et al., 2014), which would preclude the formation of UNC5 homodimers. Therefore UNC5-dependent, DCC-independent short-range axon repulsion in response to netrin-1 may require dimerization with additional receptors such as DSCAM, discussed later in this review. Alternatively, high concentrations of netrin-1 may saturate DCC, and prevent DCC homodimerization and attractive responsiveness.
Switch 4: Extracellular Environment
Surprisingly little is known regarding modification of netrin-1 attraction and repulsion by the composition of the extracellular environment. One other axon guidance molecule, draxin, has been shown to antagonize netrin-1 and is proposed to render netrin-1 a fasciculation cue (Gao et al., 2015; Liu et al., 2018), however, any role in mediating netrin-1 attraction or repulsion is unknown. Additionally, netrin-1 is known to bind to glycosaminoglycans (GAGs), a major component of the extracellular matrix, yet once again the role of these glycoproteins in axon guidance is unknown. Draxin and GAGs will be discussed in more detail later in this review. The integrin ligand laminin-1 is one of the few known extracellular components that switches netrin-1 dependent attraction to repulsion. Xenopus retinal growth cones, normally attracted to netrin-1, will be repulsed if laminin-1 is present in the extracellular matrix (ECM) or substrate (Höpker et al., 1999). This response is also likely sensitive to secondary messengers, as in the presence of substrate-adhered laminin-1, blockade of Ca2+ release, which is dependent on Ca2+-calmodulin-dependent protein kinase II (CaMKII), calcineurin (CaN) and protein phosphatase 1 (PP1), switches a repulsive netrin-1 response to attraction in Xenopus spinal neurons (Wen et al., 2004). This suggests an intriguing modulation of internal signaling by extracellular matrix components; indeed in chick ciliary ganglion neurons, treatment with laminin induces an influx of Ca2+ (Bixby et al., 1994). Whereas a Ca2+ influx blockade can switch netrin-1 attraction to repulsion (Hong et al., 2000), a further increase of Ca2+ beyond the attractive netrin-1 response regime may switch the response once more. The integration of signaling events from a complex extracellular environment represents a rich area for future studies into axon guidance. The requirement for substrate adhesion of laminin-1 may also indicate that the netrin-1 attractive-repulsive switch is in part reliant on mechanotransduction pathways, however, this remains to be investigated. This is an especially intriguing area for study of mechanical regulation of axon guidance, as many mechanotransduction proteins are known to be ion channels, which could modify the intracellular environment (Benavides Damm and Egli, 2014; Piperi and Basdra, 2015; Geng et al., 2017).
Chemotaxis and Haptotaxis?
The dogma of the field has long posited that netrin-1 is a diffusible cue, supported by assays in which axons extend from explants toward netrin-1 secreted by distant patches of cells (Tessier-Lavigne et al., 1988; Kennedy et al., 1994; Serafini et al., 1994; Yamauchi et al., 2017; Xu et al., 2018) or diffusing from enriched agarose blocks (Xu et al., 2018), and by the presence of a gradient of netrin-1 protein in embryonic chick spinal cord (Kennedy et al., 2006). However, a new axis recently emerged for netrin-1 during haptotactic axon guidance. Netrin-1 binds the extracellular matrix (Kennedy et al., 1994) or cell membranes (Kennedy et al., 2006) and guides axons locally (Deiner et al., 1997; Brankatschk and Dickson, 2006), supporting a potential role for netrin as a haptotactic cue that promotes mechanotransduction. When beads covalently linked to netrin-1 are presented to an extending spinal commissural axon in vitro, the growth cone exerts force on the bead (Moore et al., 2009). If the bead is immobilized, growth cones reorient toward the bead. Adhesion of netrin-1 to the substrate is suggested to be necessary for attractive axon guidance in spinal commissural neurons, as inhibiting netrin adhesion with heparin blocks this attractive response, and deletion of the highly positively charged C-terminal extracellular matrix-binding C domain of netrin-1 reduces axon outgrowth (Moore et al., 2012). This attraction to adhesive netrin involves non-muscle myosin II (MyoII)-dependent mechanotransduction, as blebbistatin treatment blocked the generation of forces on netrin-1 beads by the growth cone. Indeed, netrin-1 signaling through DCC activates MyoII via indirect activation of myosin light-chain kinase (MLCK) (Murray et al., 2010). MyoII also promotes mechanical activation of focal adhesion kinase (FAK), an important downstream effector of netrin-1 signaling through DCC (Moore et al., 2012) (covered in more detail in a later section). With these several pieces of evidence supporting a haptotactic response to netrin-1, now the relative contributions of haptotactic vs. chemotactic responses to netrin need to be revisited. Fragments of netrin-1 that lack what are considered the major ECM-binding domains are able to produce axon outgrowth responses (Moore et al., 2012) despite reduced interaction with the substrate, suggestive of potential adhesion-independent, chemotactic effects of netrin-1 on the axon. Whether these fragments maintain residual substrate binding that mediates haptotactic axon reorientation remains to be shown.
The original studies on the function of netrin-1 in vivo relied on a hypomorphic gene trap allele of Ntn1 that maintained low levels of netrin-1 protein (Serafini et al., 1996), however, recent development of mice carrying a floxed Ntn1 allele has allowed for tissue-specific and complete loss of netrin-1 (Bin et al., 2015; Yung et al., 2015). Three recent papers have galvanized the potential role of netrin-1 as a haptotactic cue in vivo by selectively deleting netrin-1 from the floor plate and/or ventricular zone of the spine and hindbrain using floxed Ntn1 alleles, and assessing the midline crossing of commissural axons (Dominici et al., 2017; Varadarajan et al., 2017; Yamauchi et al., 2017) (for an additional mini-review on Dominici et al. and Varadarajan et al., see Morales, 2018). All three groups found that netrin-1 expression in the floor plate, originally thought to be the source of the attractive gradient of netrin-1 responsible for commissural crossing (Maclennan et al., 1997; Kennedy et al., 2006), is not necessary for commissure formation. Rather, netrin-1 secreted by ventricular zone neural progenitors is deposited on the pial surface and forms a path for axons to reach the site of the commissure (Dominici et al., 2017; Varadarajan et al., 2017). Netrin-1 deposition on the extending axons occurs in a DCC-dependent manner (Varadarajan et al., 2017). Presentation of netrin-1 on these axons could be maintained where it is initially bound by DCC, as the growth cone continues to extend beyond these sites, or alternatively could be distributed down the axon by retrograde transport of netrin-1/DCC from the growth cone (Moore et al., 2009). Although deposition of netrin-1 on a surface supports a haptotactic guidance role, it does not invalidate earlier experiments showing a chemotactic function of netrin-1, since DCC-dependent deposition suggests that netrin-1 may not be substrate-adhered at all times. These experiments together question whether netrin-1 functions as a chemotactic cue, and potentially suggest that netrin-1 may signal differently through chemotactic and haptotactic mechanisms, or perhaps that other modulators of netrin-1 guidance are critical in situ. Clearly further experiments are required to determine the role of chemotactic and haptotactic netrin-1 responses in vitro and in vivo, however, the data accumulated over the past 30 years suggest that both mechanisms are important during nervous system development.
Netrin-1 Receptors and their Mechanisms
Netrin-1 exerts its influence through a diverse array of membrane-spanning receptors. These receptors possess extracellular domains that specifically bind netrin and cytoplasmic domains that interact with effector proteins. The cast of receptors includes DCC (Frazzled in Drosophila, Unc-40 in C. elegans), its vertebrate paralog neogenin, the UNC5 family (Unc5/Unc-5 in both Drosophila and C. elegans), and DSCAM (DSCAM in Drosophila and C. elegans).
Deleted in Colorectal Cancer (DCC)
Deleted in colorectal cancer is a transmembrane receptor of the immunoglobulin superfamily highly expressed in spinal commissural neurons (Keino-Masu et al., 1996), the retina (Gad et al., 2000; Johansson et al., 2001), and many projection neurons of the fore- and midbrain during embryonic development (Shu et al., 2000). DCC functions as a receptor for netrin-1 in both growth cone attraction and repulsion (Hamelin et al., 1993; Chan et al., 1996; Keino-Masu et al., 1996; Kolodziej et al., 1996; Fazeli et al., 1997; Colavita and Culotti, 1998; Finci et al., 2014). The extracellular portion of DCC consists of four Ig-like N-terminal domains followed by six fibronectin type-III (FN3) repeats (Keino-Masu et al., 1996). Structural studies reveal that netrin-1 binds in the area of the fifth and sixth FN3 domains, and that attractive axon guidance requires binding to two of these sites (Geisbrecht et al., 2003; Mille et al., 2009; Finci et al., 2014). The binding of a single molecule of netrin-1 to two receptors induces DCC homodimerization (Finci et al., 2014). As there are at least three binding sites on DCC for netrin-1, and three binding sites on netrin-1 for DCC (Finci et al., 2014, 2015; Xu et al., 2014), netrin-1 may link dimers to produce the larger-order clustering of DCC observed in vitro (Matsumoto and Nagashima, 2010; Xu et al., 2014; Gopal et al., 2016; Plooster et al., 2017). A similar clustering process increases adhesion avidity in the integrin/laminin receptor/ligand system (Carman and Springer, 2003; Iwamoto and Calderwood, 2015), and could represent a mechanism for the regulation of mechanical forces on the netrin-1/DCC complex.
The increase in receptor valence due to netrin-1:DCC multimerization likely brings the intracellular domains of the receptors into close apposition (Finci et al., 2014). The intracellular region of DCC contains three domains termed P1, P2, and P3, which are conserved among DCC family proteins (Kolodziej et al., 1996). When multiple DCC molecules coalesce due to netrin-1-dependent clustering, the P3 domains interact (Stein et al., 2001), and these binding-site-rich intracellular domains form a scaffold for the recruitment of downstream effectors and regulatory proteins (Li et al., 2002a). As the intracellular domains of DCC are required for repulsive netrin-1 dependent axon responses as well as attraction, parsing discrete downstream signaling pathways is complicated. This section specifically addresses mechanisms associated with either attractive axon guidance or increased axon outgrowth in response to netrin-1 downstream of DCC. Asymmetrical changes in the shape and rate of extension of the growth cone reorient outgrowth during turning; this involves dramatic and regulated remodeling of the plasma membrane (Quiroga et al., 2017) and underlying cytoskeleton (Dent et al., 2011), which is orchestrated by both chemical and mechanical transduction downstream of netrin/DCC, summarized in Figure 5.
DCC Interactions With the Cytoskeleton
The P3 domain of DCC is a hotspot for interaction with many binding partners of DCC that are poised to promote cytoskeletal and membrane remodeling, including the unconventional myosin X (MyoX), the non-receptor tyrosine kinase FAK, the E3 ubiquitin ligase TRIM9, F-actin binding ezrin-radixin-moesin (ERM) proteins, and p120RasGAP, which are situated to modulate chemical signaling, mechanotransduction, or both. The MyTH4-FERM domain of MyoX binds to the P3 domain of DCC and to microtubules, whereas the head/motor domain of MyoX translocates along filamentous actin; as such, MyoX translocates DCC to the periphery of cells and tips of filopodia (Zhu et al., 2007; Wei et al., 2011). MyoX is also required for netrin-1 dependent axon outgrowth and guidance of spinal commissural neurons (Zhu et al., 2007). Now that conditional alleles for MyoX exist (Heimsath et al., 2017), exploring the roles of MyoX in netrin dependent mechanotransduction or the formation of specific axon tracts in the brain is possible. DCC also reciprocally regulates MyoX, increasing association with actin filaments and promoting filopodial formation (Liu et al., 2012). The modulation of MyoX localization, and potentially function, by DCC represents an intriguing direction for future studies into the effect of extracellular ligands on intracellular force generation, however, future studies need to confirm the netrin dependency of MyoX enhanced actin binding.
Netrin-dependent remodeling of the actin and microtubule cytoskeletons are critical points in axon guidance that may also be regulated by mechanotransduction. The formation of filopodia in netrin-1 dependent axon guidance relies on the Ena/VASP family of actin polymerases (Gitai et al., 2003; Lebrand et al., 2004) which, along with DCC, localize to the tips of growth cone filopodia (Lanier et al., 1999; Shekarabi and Kennedy, 2002; Applewhite et al., 2007; Gupton and Gertler, 2007; Menon et al., 2015). Increases in the length and number of filopodia involves PKA phosphorylation of Mena at S236, corresponding to S157 in VASP (Lebrand et al., 2004; Deming et al., 2015). VASP, but not Mena or the third Ena/VASP family member EVL, is also regulated in netrin-1 dependent axon guidance by non-degradative TRIM9-dependent ubiquitination ([Menon et al., 2015